REVLIMID Drug Patent Profile
✉ Email this page to a colleague
When do Revlimid patents expire, and what generic alternatives are available?
Revlimid is a drug marketed by Bristol Myers Squibb and is included in one NDA. There are three patents protecting this drug and three Paragraph IV challenges.
This drug has three hundred and thirty-one patent family members in forty-one countries.
The generic ingredient in REVLIMID is lenalidomide. There are fourteen drug master file entries for this compound. Thirteen suppliers are listed for this compound. Additional details are available on the lenalidomide profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Revlimid
A generic version of REVLIMID was approved as lenalidomide by ARROW INTL on May 21st, 2021.
Summary for REVLIMID
| International Patents: | 331 |
| US Patents: | 3 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 149 |
| Clinical Trials: | 546 |
| Patent Applications: | 633 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for REVLIMID |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for REVLIMID |
| What excipients (inactive ingredients) are in REVLIMID? | REVLIMID excipients list |
| DailyMed Link: | REVLIMID at DailyMed |

Recent Clinical Trials for REVLIMID
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| BeiGene | Phase 2 |
| SWOG Cancer Research Network | Phase 2 |
| Ipsen | Phase 2 |
Pharmacology for REVLIMID
| Drug Class | Thalidomide Analog |
Anatomical Therapeutic Chemical (ATC) Classes for REVLIMID
Paragraph IV (Patent) Challenges for REVLIMID
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| REVLIMID | Capsules | lenalidomide | 2.5 mg and 20 mg | 021880 | 1 | 2016-07-12 |
| REVLIMID | Capsules | lenalidomide | 5 mg, 10 mg and 15 mg | 021880 | 1 | 2010-08-30 |
| REVLIMID | Capsules | lenalidomide | 25 mg | 021880 | 1 | 2010-07-12 |
US Patents and Regulatory Information for REVLIMID
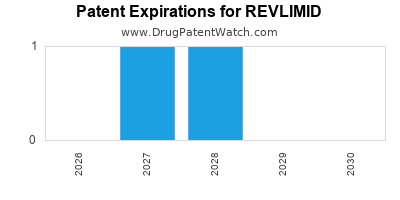
REVLIMID is protected by three US patents and three FDA Regulatory Exclusivities.
Patents protecting REVLIMID
Polymorphic forms of 3-(4-amino-1-oxo-1,3 dihydro-isoindol-2-yl)-piperidine-2,6-dione
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Polymorphic forms of 3-(4-amino-1-oxo-1,3 dihydro-isoindol-2-yl)-piperidine-2,6-dione
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of mantle cell lymphomas
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF REVLIMID (LENALIDOMIDE) FOR THE TREATMENT OF PATIENTS WITH MANTLE CELL LYMPHOMA WHOSE DISEASE HAS RELAPSED OR PROGRESSED AFTER TWO PRIOR THERAPIES, ONE OF WHICH INCLUDED BORTEZOMIB
FDA Regulatory Exclusivity protecting REVLIMID
TREATMENT OF MULTIPLE MYELOMA (MM), AS MAINTENANCE FOLLOWING AUTOLOGOUS HEMATOPOIETIC STEM CELL TRANSPLANTATION (AUTO-HSCT)
Exclusivity Expiration: ⤷ Try a Trial
INDICATED IN COMBINATION WITH A RITUXIMAB PRODUCT FOR THE TREATMENT OF ADULT PATIENTS WITH PREVIOUSLY TREATED FOLLICULAR LYMPHOMA (FL)
Exclusivity Expiration: ⤷ Try a Trial
INDICATED IN COMBINATION WITH A RITUXIMAB PRODUCT FOR THE TREATMENT OF ADULT PATIENTS WITH PREVIOUSLY TREATED MARGINAL ZONE LYMPHOMA (MZL)
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-004 | Jun 29, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-006 | Jun 5, 2013 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-004 | Jun 29, 2006 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-006 | Jun 5, 2013 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for REVLIMID
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-003 | Jun 29, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-004 | Jun 29, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-003 | Jun 29, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-003 | Jun 29, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for REVLIMID
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Ireland Limited | Lenalidomide Mylan | lenalidomide | EMEA/H/C/005306 Multiple myelomaLenalidomide Mylan as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.Lenalidomide Mylan as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.Lenalidomide Mylan in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.Follicular lymphomaLenalidomide Mylan in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1-3a). |
Authorised | yes | no | no | 2020-12-18 | |
| Accord Healthcare S.L.U. | Lenalidomide Accord | lenalidomide | EMEA/H/C/004857 Multiple myelomaLenalidomide Accord as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.Lenalidomide Accord as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4.2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.Lenalidomide Accord in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.Follicular lymphomaLenalidomide Accord in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 – 3a). |
Authorised | yes | no | no | 2018-09-20 | |
| Bristol-Myers Squibb Pharma EEIG | Revlimid | lenalidomide | EMEA/H/C/000717 Multiple myelomaRevlimid as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.Revlimid as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4.2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.Revlimid in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.Myelodysplastic syndromesRevlimid as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.Mantle cell lymphomaRevlimid as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma.Follicular lymphomaRevlimid in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 – 3a). |
Authorised | no | no | no | 2007-06-14 | |
| Krka, d.d., Novo mesto | Lenalidomide Krka (previously Lenalidomide Krka d.d. Novo mesto) | lenalidomide | EMEA/H/C/005348 Multiple myelomaLenalidomide Krka as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.Lenalidomide Krka as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4.2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.Lenalidomide Krka in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.Myelodysplastic syndromesLenalidomide Krka as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.Mantle cell lymphomaLenalidomide Krka as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (see sections 4.4 and 5.1).Follicular lymphomaLenalidomide Krka in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 – 3a).Multiple myelomaLenalidomide Krka as monotherapy is indicated for the maintenance treatment of adult patients with newly diagnosed multiple myeloma who have undergone autologous stem cell transplantation.Lenalidomide Krka as combination therapy with dexamethasone, or bortezomib and dexamethasone, or melphalan and prednisone (see section 4.2) is indicated for the treatment of adult patients with previously untreated multiple myeloma who are not eligible for transplant.Lenalidomide Krka in combination with dexamethasone is indicated for the treatment of multiple myeloma in adult patients who have received at least one prior therapy.Myelodysplastic syndromesLenalidomide Krka as monotherapy is indicated for the treatment of adult patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q cytogenetic abnormality when other therapeutic options are insufficient or inadequate.Mantle cell lymphomaLenalidomide Krka as monotherapy is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (see sections 4.4 and 5.1).Follicular lymphomaLenalidomide Krka in combination with rituximab (anti-CD20 antibody) is indicated for the treatment of adult patients with previously treated follicular lymphoma (Grade 1 – 3a). |
Authorised | yes | no | no | 2021-02-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for REVLIMID
See the table below for patents covering REVLIMID around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20140082857 | METHODS USING 3-(4-AMINO-1-OXO-1,3-DIHYDRO-ISOINDOL-2-YL)- PIPERIDINE-2,6-DIONE FOR TREATMENT OF CERTAIN LEUKEMIAS | ⤷ Try a Trial |
| Japan | 2013091659 | METHOD USING 3-(4-AMINO-1-OXO-1,3-DIHYDRO-ISOINDOL-2-YL)-PIPERIDINE-2,6-DIONE FOR TREATMENT OF CERTAIN LEUKEMIA | ⤷ Try a Trial |
| Norway | 336898 | ⤷ Try a Trial | |
| Japan | 2013526525 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for REVLIMID
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0925294 | 91359 | Luxembourg | ⤷ Try a Trial | 91359, EXPIRES: 20220614 |
| 2105135 | C20150005 00140 | Estonia | ⤷ Try a Trial | PRODUCT NAME: POMALIDOMIID;REG NO/DATE: EU/1/13/850 08.08.2013 |
| 2105135 | 122015000013 | Germany | ⤷ Try a Trial | PRODUCT NAME: POMALIDOMID UND SEINE PHARMAZEUTISCH AKZEPTABLEN SALZE, SOLVATE, HYDRATE ODER STEREOISOMERE (IMNOVID); REGISTRATION NO/DATE: EU/1/13/850 20130805 |
| 2105135 | 00140 | Estonia | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


