LASTACAFT Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Lastacaft, and when can generic versions of Lastacaft launch?
Lastacaft is a drug marketed by Abbvie and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has forty-six patent family members in thirty countries.
The generic ingredient in LASTACAFT is alcaftadine. There are six drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the alcaftadine profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Lastacaft
A generic version of LASTACAFT was approved as alcaftadine by GLAND PHARMA LTD on March 1st, 2024.
Summary for LASTACAFT
| International Patents: | 46 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 70 |
| Clinical Trials: | 5 |
| Patent Applications: | 155 |
| Drug Prices: | Drug price information for LASTACAFT |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for LASTACAFT |
| What excipients (inactive ingredients) are in LASTACAFT? | LASTACAFT excipients list |
| DailyMed Link: | LASTACAFT at DailyMed |
Recent Clinical Trials for LASTACAFT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Allergan | |
| ORA, Inc. | Phase 4 |
| Starx Research Center, LLC | Phase 4 |
Pharmacology for LASTACAFT
| Drug Class | Histamine-1 Receptor Antagonist |
| Mechanism of Action | Histamine H1 Receptor Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for LASTACAFT
Paragraph IV (Patent) Challenges for LASTACAFT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| LASTACAFT | Ophthalmic Solution | alcaftadine | 0.25% | 022134 | 1 | 2014-07-30 |
US Patents and Regulatory Information for LASTACAFT
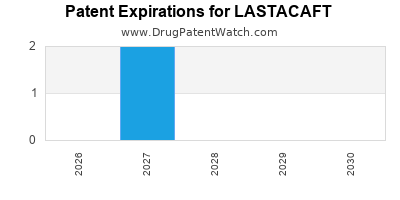
LASTACAFT is protected by two US patents.
Patents protecting LASTACAFT
Ophthalmic compositions containing alcaftadine
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF LASTACAFT TO TEMPORARY RELIEVE ITCHY EYES DUE TO POLLEN, RAGWEED, GRASS, ANIMAL HAIR AND DANDER
Ocular allergy treatments with alcaftadine
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF LASTACAFT TO TEMPORARY RELIEVE ITCHY EYES DUE TO POLLEN, RAGWEED, GRASS, ANIMAL HAIR AND DANDER
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | LASTACAFT | alcaftadine | SOLUTION/DROPS;OPHTHALMIC | 022134-001 | Jul 28, 2010 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | LASTACAFT | alcaftadine | SOLUTION/DROPS;OPHTHALMIC | 022134-001 | Jul 28, 2010 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for LASTACAFT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | LASTACAFT | alcaftadine | SOLUTION/DROPS;OPHTHALMIC | 022134-001 | Jul 28, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for LASTACAFT
When does loss-of-exclusivity occur for LASTACAFT?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0278
Estimated Expiration: ⤷ Try a Trial
Patent: 1697
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 07234957
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0710085
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 48115
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 07000916
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1460176
Estimated Expiration: ⤷ Try a Trial
Patent: 2895234
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 414
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 04196
Estimated Expiration: ⤷ Try a Trial
Patent: 50209
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 088786
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 6221
Estimated Expiration: ⤷ Try a Trial
Patent: 0870396
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 04196
Estimated Expiration: ⤷ Try a Trial
Patent: 50209
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 31331
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 4473
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 92277
Estimated Expiration: ⤷ Try a Trial
Patent: 39716
Estimated Expiration: ⤷ Try a Trial
Patent: 09533333
Estimated Expiration: ⤷ Try a Trial
Patent: 13144703
Estimated Expiration: ⤷ Try a Trial
Patent: 15131820
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 58
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 3669
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 08012657
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 1690
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 0800261
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 1147
Estimated Expiration: ⤷ Try a Trial
Patent: 084593
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 080053
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 04196
Estimated Expiration: ⤷ Try a Trial
Patent: 50209
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 0044
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0809327
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1321731
Estimated Expiration: ⤷ Try a Trial
Patent: 080110881
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 94655
Estimated Expiration: ⤷ Try a Trial
Patent: 52823
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 50721
Estimated Expiration: ⤷ Try a Trial
Patent: 78990
Estimated Expiration: ⤷ Try a Trial
Patent: 0815016
Estimated Expiration: ⤷ Try a Trial
Patent: 1446249
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 938
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 254
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering LASTACAFT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 101321731 | ⤷ Try a Trial | |
| Chile | 2007000916 | Uso de alcaftadina y de acido 6,11-dihidro-11-(1-metil-4-piperidiniliden)-5h-imidazo[2,1-b][3]benzazepin-3-carboxilico, sales, n-oxidos, solvatos e hidratos para tratar sintomas de alergia ocular, inflamacion ocular y sintomas nasales de alergia ocular; composiciones oftalmicas que los comprenden; su uso y kit farmaceutico. | ⤷ Try a Trial |
| Australia | 2007234957 | Ocular allergy treatments | ⤷ Try a Trial |
| Poland | 170771 | ⤷ Try a Trial | |
| New Zealand | 242774 | SUBSTITUTED IMIDAZO[2,1-B][3]BENZAZEPINE DERIVATIVES, PREPARATION AND PHARMACEUTICAL COMPOSITIONS THEREOF | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


