CYCLOSET Drug Patent Profile
✉ Email this page to a colleague
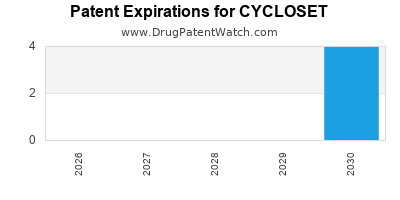
When do Cycloset patents expire, and when can generic versions of Cycloset launch?
Cycloset is a drug marketed by Veroscience and is included in one NDA. There are twelve patents protecting this drug.
This drug has thirty-two patent family members in thirteen countries.
The generic ingredient in CYCLOSET is bromocriptine mesylate. There are nine drug master file entries for this compound. Eight suppliers are listed for this compound. Additional details are available on the bromocriptine mesylate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Cycloset
A generic version of CYCLOSET was approved as bromocriptine mesylate by SANDOZ on January 13th, 1998.
Summary for CYCLOSET
| International Patents: | 32 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 54 |
| Clinical Trials: | 9 |
| Patent Applications: | 3,142 |
| Formulation / Manufacturing: | see details |
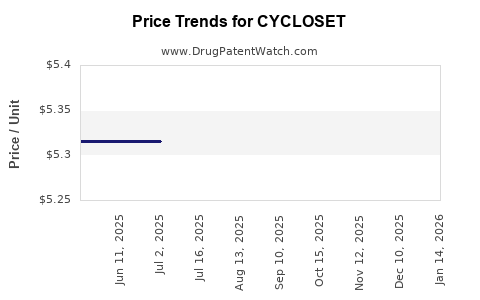
| Drug Prices: | Drug price information for CYCLOSET |
| What excipients (inactive ingredients) are in CYCLOSET? | CYCLOSET excipients list |
| DailyMed Link: | CYCLOSET at DailyMed |
Recent Clinical Trials for CYCLOSET
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Dennis McNamara | Phase 4 |
| National Heart, Lung, and Blood Institute (NHLBI) | Phase 4 |
| Jaime Daniel Mondragon | Phase 2/Phase 3 |
Pharmacology for CYCLOSET
| Drug Class | Ergot Derivative |
Anatomical Therapeutic Chemical (ATC) Classes for CYCLOSET
US Patents and Regulatory Information for CYCLOSET
CYCLOSET is protected by one hundred and twenty-two US patents.
Patents protecting CYCLOSET
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1 AND 2 AND 3
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 4 AND 5
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 7, 8, AND 9
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1 AND 2
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 4, 5, AND 6
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 7 AND 8
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 10 AND 11
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 16, 17, AND 18
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 13 AND 14
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 10, 11, AND 12
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 16 AND 17
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 13, 14, AND 15
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 28 AND 29
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 25 AND 26
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 22, 23, AND 24
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 19 AND 20
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 19, 20, AND 21
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 28, 29, AND 30
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 25, 26, AND 27
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 1 AND 10
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 19 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 16 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 2 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 30 WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 27 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 17 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 15 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 28 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 3 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 8 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 5 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 1 AND 11
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1, 17 AND 18 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 1 AND 13
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 22 WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 22
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 22 AND 24 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 22
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 29 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 6 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 26 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 4 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1 AND 7 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 22 AND 25 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 22
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 1 AND 9
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 22 AND 23 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 22
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 56 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 31 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 44 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 44 AND 54
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 50 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO TREAT GLUCOSE INTOLERANCE IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 42 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 42
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 44 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 49 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 45 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 34 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 51 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 32 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 38 AND WHEREIN THE EFFECTS ARE AS RECITEDCLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 39 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ONCE DAILY TOPICAL TREATMENT OF PERSISTENT FACIAL ERYTHEMA ASSOCIATED WITH ROSACEA IN ADULTS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 36 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 48 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 33 WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO TREAT GLUCOSE INTOLERANCE IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 42 AND 43 WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 42
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 47 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 26 AND 40 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 26
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 57 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 46 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 44
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 44 AND 52 WHEREIN THE EFFECTS ARE AS RECITED IN SAID CLAIMS
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1 AND 11
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 13 AND 23
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1, 11, AND 12
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETIS BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 13, 23, AND 24
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: IMPROVEMENT OF GLYCEMIC CONTROL IN INDIVIDUALS WITH TYPE 2 DIABETES
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: IMPROVEMENT OF GLYCEMIC CONTROL IN INDIVIDUALS WITH TYPE 2 DIABETES
Combination of dopamine agonists plus first phase secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE WHEREIN THE COMBINED THERAPEUTIC EFFECT IS GREATER THAN THE ADDITIVE EFFECT OF ADMINISTERING EACH AGENT ALONE
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: IMPROVEMENT OF GLYCEMIC CONTROL IN INDIVIDUALS WITH TYPE 2 DIABETES
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 1-5 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 1-5
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 7 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 7
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO TREAT GLUCOSE INTOLERANCE IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 14-15 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 14-15
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 9 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 9
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO TREAT GLUCOSE INTOLERANCE IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 16-18 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 16-18
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 10 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 10
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 6 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 6
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 19 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 19
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 12 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 12
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADMINISTRATION ONCE DAILY WITHIN TWO HOURS AFTER WAKING IN THE MORNING FOR IMPROVEMENT OF GLYCEMIC CONTROL IN A TYPE 2 DIABETES PATIENT
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: IMPROVEMENT OF GLYCEMIC CONTROL IN INDIVIDUALS WITH TYPE 2 DIABETES
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 41, 52, AND 53
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 66, 75, AND 76
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 29, 39, AND 40
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1, 13, AND 14
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 54 AND 64
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 29 AND 39
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1 AND 13
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 66 AND 75
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 41 AND 52
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 89, 99, AND 100
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 54, 64, AND 65
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 15, 27, AND 28
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 15 AND 27
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 77 AND 87
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 89 AND 99
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ORALLY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 77, 87, AND 88
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 11 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 11
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO TREAT GLUCOSE INTOLERANCE IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 2 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 2
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 16-19 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 16-19
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 12 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 12
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 9 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 9
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 8 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 8
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 14 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 14
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIM 1 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIM 1
Combination of dopamine agonists plus first phase insulin secretagogues for the treatment of metabolic disorders
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING BROMOCRIPTINE MESYLATE AND A FIRST-PHASE INSULIN SECRETAGOGUE AS RECITED IN CLAIMS 3-7 AND WHEREIN THE EFFECTS ARE AS RECITED IN CLAIMS 3-7
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 43, 50 AND 51
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1,10 AND 11
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 30, 41, AND 42
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING MICRONIZED BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 1 AND 10
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 30 AND 41
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 21 AND 28
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 12 AND 19
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 43 AND 50
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 12, 19 AND 20
Bromocriptine formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES BY ADMINISTERING A DOSAGE FORM COMPRISING BROMOCRIPTINE AND ONE OR MORE EXCIPIENTS AS RECITED IN CLAIMS 21, 28, AND 29
Expired US Patents for CYCLOSET
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Veroscience | CYCLOSET | bromocriptine mesylate | TABLET;ORAL | 020866-001 | May 5, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for CYCLOSET
See the table below for patents covering CYCLOSET around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2009046504 | USE OF PROLACTIN-DECREASING AGENT AND/OR PROLACTIN-INCREASING AGENT FOR REGULATION OF IMMUNE FUNCTION | ⤷ Try a Trial |
| Uruguay | 34776 | FORMULACIONES DE BROMOCRIPTINA | ⤷ Try a Trial |
| Brazil | 9206190 | ⤷ Try a Trial | |
| European Patent Office | 0764026 | ⤷ Try a Trial | |
| Hungary | 9603543 | ⤷ Try a Trial | |
| European Patent Office | 0591458 | ⤷ Try a Trial | |
| South Africa | 9410263 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


