Teva Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA, and when can generic versions of TEVA drugs launch?
TEVA has seven hundred and thirty-six approved drugs.
There are one hundred US patents protecting TEVA drugs. There are fifty-three tentative approvals on TEVA drugs.
There are nine hundred and thirteen patent family members on TEVA drugs in forty-nine countries and one thousand and fifty-six supplementary protection certificates in eighteen countries.
Summary for Teva
| International Patents: | 913 |
| US Patents: | 100 |
| Tradenames: | 508 |
| Ingredients: | 443 |
| NDAs: | 736 |
| Patent Litigation for Teva: | See patent lawsuits for Teva |
| PTAB Cases with Teva as petitioner: | See PTAB cases with Teva as petitioner |
Drugs and US Patents for Teva
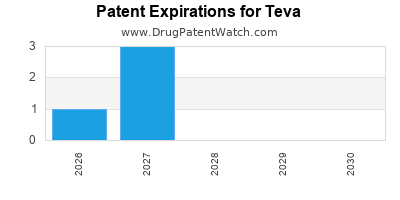
Expired US Patents for Teva
Paragraph IV (Patent) Challenges for TEVA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | for Injection | 3.5 mg/vial | ➤ Subscribe | 2016-10-26 |
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-05-17 |
| ➤ Subscribe | Extended-release Capsule | 15 mg and 30 mg | ➤ Subscribe | 2008-08-11 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-01-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 20 mg, 30 mg | ➤ Subscribe | 2009-11-18 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-02-26 |
Premature patent expirations for TEVA
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Teva Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 114177442 | ⤷ Try a Trial |
| Serbia | 58574 | ⤷ Try a Trial |
| China | 103282070 | ⤷ Try a Trial |
| European Patent Office | 2658525 | ⤷ Try a Trial |
| Denmark | 2496294 | ⤷ Try a Trial |
| Cyprus | 1113221 | ⤷ Try a Trial |
| Taiwan | 201611855 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2768484 | 384 1-2020 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: KOMBINACIA DAUNORUBICINU A CYTARABINU; REGISTRATION NO/DATE: EU/1/18/1308 20180827 |
| 0387077 | SPC/GB04/003 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PARICALCITOL; REGISTERED: ES SPAIN 64.974 20020809; UK PL 00037/0403 20030721 |
| 3150586 | 132020000000055 | Italy | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT O UN SALE FARMACOLOGICAMENTE ACCETTABILE O UN SOLVATO DELLO STESSO, DARUNAVIR O UN SALE FARMACOLOGICAMENTE ACCETTABILE O UN SOLVATO DELLO STESSO, IN PARTICOLARE DARUNAVIR ETANOLATO, E EMTRICITABINE O UN SALE FARMACOLOGICAMENTE ACCETTABILE O UN SOLVATO DELLO STESSO(SYMTUZA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/17/1225, 20170925 |
| 2443246 | 2021C/538 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VAZKEPA - ICOSAPENT ETHYL; AUTHORISATION NUMBER AND DATE: EU/1/20/1524 20210329 |
| 2269604 | 122016000094 | Germany | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUS ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/09/538/001-006 20090803 |
| 0810209 | 07C0034 | France | ⤷ Try a Trial | PRODUCT NAME: DARUNAVIR; REGISTRATION NO/DATE: EU/1/06/380/001 20070212 |
| 0166287 | 96C0032 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PANTOPRAZOL. NATR. SESQUIHYDRAS PANTOPRAZOLE; NAT. REGISTRATION NO/DATE: 127 IS 98 F 3 19960222; FIRST REGISTRATION: SE 12131 19940506 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.