Sandoz Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANDOZ, and what generic alternatives to SANDOZ drugs are available?
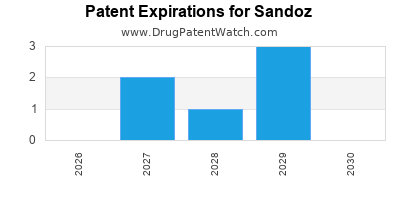
SANDOZ has four hundred and eighty-nine approved drugs.
There are nine US patents protecting SANDOZ drugs. There are thirty-three tentative approvals on SANDOZ drugs.
There are one hundred and three patent family members on SANDOZ drugs in thirty-four countries and five hundred and three supplementary protection certificates in seventeen countries.
Summary for Sandoz
| International Patents: | 103 |
| US Patents: | 9 |
| Tradenames: | 322 |
| Ingredients: | 300 |
| NDAs: | 489 |
| Patent Litigation for Sandoz: | See patent lawsuits for Sandoz |
| PTAB Cases with Sandoz as petitioner: | See PTAB cases with Sandoz as petitioner |
Drugs and US Patents for Sandoz
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sandoz | GUANFACINE HYDROCHLORIDE | guanfacine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 202568-003 | Jun 3, 2015 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | NAPROXEN SODIUM | naproxen sodium | TABLET;ORAL | 074162-002 | Dec 21, 1993 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | AMPICILLIN SODIUM | ampicillin sodium | POWDER;INTRAVENOUS | 062738-001 | Feb 19, 1987 | AP | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sandoz | GABAPENTIN | gabapentin | CAPSULE;ORAL | 075428-003 | Jan 24, 2006 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sandoz | AZOPT | brinzolamide | SUSPENSION/DROPS;OPHTHALMIC | 020816-001 | Apr 1, 1998 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sandoz
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sandoz | EXELON | rivastigmine | FILM, EXTENDED RELEASE;TRANSDERMAL | 022083-001 | Jul 6, 2007 | 5,602,176 | ⤷ Try a Trial |
| Sandoz | CILOXAN | ciprofloxacin hydrochloride | OINTMENT;OPHTHALMIC | 020369-001 | Mar 30, 1998 | 4,670,444*PED | ⤷ Try a Trial |
| Sandoz | RITALIN LA | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021284-001 | Jun 5, 2002 | 5,837,284 | ⤷ Try a Trial |
| Sandoz | FOCALIN XR | dexmethylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 021802-005 | Oct 23, 2009 | 6,635,284 | ⤷ Try a Trial |
| Sandoz | LOTREL | amlodipine besylate; benazepril hydrochloride | CAPSULE;ORAL | 020364-004 | Mar 3, 1995 | 6,162,802 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANDOZ drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release capsules | 25 mg | ➤ Subscribe | 2011-09-30 |
| ➤ Subscribe | Extended-release Tablets | 80 mg | ➤ Subscribe | 2007-03-15 |
| ➤ Subscribe | Extended-release Capsules | 10 mg | ➤ Subscribe | 2007-05-21 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2004-05-27 |
| ➤ Subscribe | Capsules | 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg and 10 mg/20 mg | ➤ Subscribe | 2004-06-09 |
| ➤ Subscribe | Ophthalmic Emulsion | 0.05% | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Oral Solution | 2 mg/mL | ➤ Subscribe | 2004-11-05 |
| ➤ Subscribe | Injection | 100 mg/mL, 2.5 mL vials | ➤ Subscribe | 2007-09-24 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Oral Solution | 4 mg/5 mL | ➤ Subscribe | 2004-12-20 |
| ➤ Subscribe | Extended-release Capsules | 5mg, 10mg and 20 mg | ➤ Subscribe | 2007-03-30 |
| ➤ Subscribe | Injection | 0.05 mg/mL, 100 mL vial | ➤ Subscribe | 2008-08-29 |
| ➤ Subscribe | Extended-release Capsule | 40 mg | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Otic Suspension | 0.3%/0.1% | ➤ Subscribe | 2012-07-31 |
| ➤ Subscribe | Extended-release capsules | 35 mg | ➤ Subscribe | 2011-09-29 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2004-07-27 |
| ➤ Subscribe | Extended-release Capsules | 20 mg, 30 mg and 40 mg | ➤ Subscribe | 2006-08-21 |
| ➤ Subscribe | Capsules | 5 mg/40 mg and 10 mg/40 mg | ➤ Subscribe | 2006-11-17 |
| ➤ Subscribe | Capsules | 1.5 mg, 3 mg, 4.5 mg and 6 mg | ➤ Subscribe | 2004-04-21 |
| ➤ Subscribe | Transdermal System Extended-release | 13.3 mg/24 hr | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Injection | 1 mg/mL, 50 mL vials | ➤ Subscribe | 2011-12-16 |
| ➤ Subscribe | Extended-release Capsules | 15 mg | ➤ Subscribe | 2007-05-14 |
| ➤ Subscribe | For Injection | 250 mg/vial | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Extended-release Capsule | 30 mg | ➤ Subscribe | 2010-12-15 |
| ➤ Subscribe | Ophthalmic Solution | 0.00% | ➤ Subscribe | 2009-02-19 |
International Patents for Sandoz Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Taiwan | 200530250 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 03026671 | ⤷ Try a Trial |
| Taiwan | I345977 | ⤷ Try a Trial |
| South Africa | 200901164 | ⤷ Try a Trial |
| China | 101522171 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sandoz Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1411900 | 18/2011 | Austria | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN UND ESOMEPRAZOL SOWIE DEREN PHARMAZEUTISCH ANNEHMBARE SALZE; NAT. REGISTRATION NO/DATE: 1-29937 20110105; FIRST REGISTRATION: GB PL 17901/0263-0001 20101105 |
| 0509752 | SPC/GB99/043 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DORZOLAMIDE OR AN OPHTHALMOLOGICALLY ACCEPTABLE SALT THEREOF, PREFERABLY DORZOLAMIDE HYDROCHOLORIDE, PLUS TIMOLOL OR AN OPHTHAMOLOGICALLY ACCEPTABLE SALT THEREOF, PREFERABLY TIMOLOL MALEATE; REGISTERED: DK 19045 19980306; UK PL 00025/0373 19980804 |
| 0454436 | C970015 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: OLANZAPINE, DESGEWENST IN DE VORM VAN EEN ZUURADDITIEZOUT; REGISTRATION NO/DATE: EU/1/96/022/001 - EU/1/96/022/010 19960927 |
| 0961612 | 2009C/046 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL ALBUMIN; AUTHORISATION NUMBER AND DATE: EU/1/07/428/001 20080111 |
| 1667986 | 92172 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: SOLVAT ACETONIQUE DU CABAZITAXEL, OU DESIGNE SOLVAT ACETONIQUE DU DIMETHOXY DOCETAXEL OU SOLVAT ACETONIQUE DU (2R,3S)-3-TERT-BUTOXYCARBONYLAMINO-2-HYDROXY-3-PHENYLPROPIONATE DE 4-ACETOXY-2A-BENZOYLOXY-5BETA,20-EPOXY-1-HYDROXY-7BETA,10A-DIMETHOXY-9-OXO-TAX-11-ENE-13A-YLE(ACETONATE DU CABAZITAXEL) |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.