Emd Serono Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for EMD SERONO INC, and when can generic versions of EMD SERONO INC drugs launch?
EMD SERONO INC has six approved drugs.
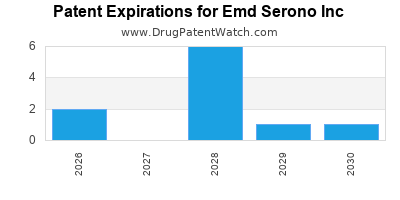
There are thirteen US patents protecting EMD SERONO INC drugs.
There are one hundred and ninety-five patent family members on EMD SERONO INC drugs in forty-two countries and eighty-nine supplementary protection certificates in sixteen countries.
Summary for Emd Serono Inc
| International Patents: | 195 |
| US Patents: | 13 |
| Tradenames: | 6 |
| Ingredients: | 5 |
| NDAs: | 6 |
| Drug Master File Entries: | 2 |
Drugs and US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Emd Serono Inc | GLUCOPHAGE | metformin hydrochloride | TABLET;ORAL | 020357-005 | Nov 5, 1998 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-002 | Sep 26, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | GLUCOPHAGE | metformin hydrochloride | TABLET;ORAL | 020357-001 | Mar 3, 1995 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | GLUCOPHAGE | metformin hydrochloride | TABLET;ORAL | 020357-004 | Nov 5, 1998 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | 6,319,192 | ⤷ Try a Trial |
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-002 | Sep 26, 1997 | 4,517,181 | ⤷ Try a Trial |
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-001 | Sep 26, 1997 | 4,517,181 | ⤷ Try a Trial |
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-002 | Aug 11, 2000 | 4,800,191 | ⤷ Try a Trial |
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-002 | Aug 11, 2000 | 6,319,192 | ⤷ Try a Trial |
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-002 | Aug 11, 2000 | 6,863,891 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Emd Serono Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Cyprus | 1119790 | ⤷ Try a Trial |
| Hong Kong | 1150266 | ⤷ Try a Trial |
| New Zealand | 583186 | ⤷ Try a Trial |
| Hong Kong | 1142891 | ⤷ Try a Trial |
| Chile | 2008001392 | ⤷ Try a Trial |
| Brazil | 112020010282 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Emd Serono Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1827461 | 18C1008 | France | ⤷ Try a Trial | PRODUCT NAME: CLADRIBINE; REGISTRATION NO/DATE: EU/1/17/1212 20170824 |
| 1506211 | 300677 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| 1827461 | 1890010-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: CLADRIBINE; REG. NO/DATE: EU/1/17/1212 20170824 |
| 0299402 | 099C0031 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ACETATE DE CETRORELIX; REGISTRATION NO/DATE: EU/1/99/100/001 19990413 |
| 1827461 | C01827461/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: CLADRIBIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66831 19.03.2019 |
| 1506211 | 42/2014 | Austria | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON DAPAGLIFLOZIN ODER EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES DAVON UND METFORMIN UND EINES PHARMAZEUTISCH VERTRAEGLICHEN SALZES DAVON; REGISTRATION NO/DATE: EU/1/13/900 (MITTEILUNG) 20140121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.