Bayer Hlthcare Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BAYER HLTHCARE, and what generic alternatives to BAYER HLTHCARE drugs are available?
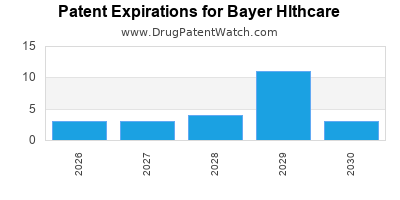
BAYER HLTHCARE has fifty-six approved drugs.
There are thirty-seven US patents protecting BAYER HLTHCARE drugs.
There are eight hundred and seven patent family members on BAYER HLTHCARE drugs in fifty-eight countries and one hundred and forty-seven supplementary protection certificates in twenty countries.
Summary for Bayer Hlthcare
| International Patents: | 807 |
| US Patents: | 37 |
| Tradenames: | 57 |
| Ingredients: | 37 |
| NDAs: | 56 |
Drugs and US Patents for Bayer Hlthcare
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | ADEMPAS | riociguat | TABLET;ORAL | 204819-003 | Oct 8, 2013 | AB | RX | Yes | No | 10,662,188 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bayer Hlthcare | STIVARGA | regorafenib | TABLET;ORAL | 203085-001 | Sep 27, 2012 | RX | Yes | Yes | 9,458,107 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bayer Hlthcare | CIPRO | ciprofloxacin | INJECTABLE;INJECTION | 019847-001 | Dec 26, 1990 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bayer Hlthcare
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bayer Hlthcare | CIPRO | ciprofloxacin hydrochloride | TABLET;ORAL | 019537-004 | Oct 22, 1987 | 4,670,444*PED | ⤷ Try a Trial |
| Bayer Hlthcare | BEYAZ | drospirenone; ethinyl estradiol; levomefolate calcium | TABLET;ORAL | 022532-001 | Sep 24, 2010 | RE37564 | ⤷ Try a Trial |
| Bayer Hlthcare | MAGNEVIST | gadopentetate dimeglumine | INJECTABLE;INJECTION | 019596-001 | Jun 2, 1988 | 4,957,939 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAYER HLTHCARE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 3 mg/0.02 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2011-11-21 |
| ➤ Subscribe | Tablets | 20 mg | ➤ Subscribe | 2009-03-05 |
| ➤ Subscribe | Tablets | 2.5 mg | ➤ Subscribe | 2009-09-04 |
| ➤ Subscribe | Tablets | 200 mg | ➤ Subscribe | 2014-02-28 |
| ➤ Subscribe | Tablets | 3 mg/0.03 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2012-09-28 |
| ➤ Subscribe | Transdermal System | 0.05 mg/day and 0.1 mg/day | ➤ Subscribe | 2005-09-12 |
| ➤ Subscribe | Tablets | 3 mg/0.02 mg | ➤ Subscribe | 2006-09-29 |
| ➤ Subscribe | Injection | 1.6 mg/mL | ➤ Subscribe | 2014-02-07 |
| ➤ Subscribe | Tablets | 0.5 mg, 1 mg,1.5 mg, 2 mg and2.5 mg | ➤ Subscribe | 2017-10-10 |
| ➤ Subscribe | Tablets | 0.25 mg/0.5 mg | ➤ Subscribe | 2015-01-08 |
| ➤ Subscribe | Tablets | 3 mg/0.02 mg/0.451 mg and 0.451 mg | ➤ Subscribe | 2012-11-13 |
| ➤ Subscribe | Tablets | 5 mg ad 10 mg | ➤ Subscribe | 2009-07-10 |
| ➤ Subscribe | Tablets | 3 mg; 2 mg/2 mg; 2 mg/3 mg; 1 mg | ➤ Subscribe | 2010-10-22 |
| ➤ Subscribe | Tablets | 25 mg, 50 mg and 100 mg | ➤ Subscribe | 2005-03-22 |
| ➤ Subscribe | Tablets | 40 mg | ➤ Subscribe | 2016-09-27 |
| ➤ Subscribe | Oral Suspension | 250 mg/5 mL and 500 mg/ 5 mL | ➤ Subscribe | 2009-10-16 |
| ➤ Subscribe | Tablets | 3 mg/0.03 mg | ➤ Subscribe | 2005-01-07 |
| ➤ Subscribe | Orally Disintegrating Tablets | 10 mg | ➤ Subscribe | 2011-12-22 |
| ➤ Subscribe | Tablets | 0.5 mg/1 mg | ➤ Subscribe | 2007-12-26 |
International Patents for Bayer Hlthcare Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 081060 | ⤷ Try a Trial |
| Russian Federation | 2018131134 | ⤷ Try a Trial |
| South Korea | 101139557 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bayer Hlthcare Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3106463 | 2020C/507 | Belgium | ⤷ Try a Trial | PRODUCT NAME: VITRAKVI - LAROTRECTINIB ET/OU SES SELS ACCEPTABLES EN PHARMACIE, EN PARTICULIER L'HYDROGENO SULFATE DE LAROTRECTINIB; AUTHORISATION NUMBER AND DATE: EU/1/19/1385 20190923 |
| 1506193 | C20140015 00109 | Estonia | ⤷ Try a Trial | PRODUCT NAME: RIOTSIGUAAT; REG NO/DATE: K(2014)2152 (LOPLIK) 31.03.2014 |
| 1140212 | 14C0038 | France | ⤷ Try a Trial | PRODUCT NAME: RADIUM 223 AINSI QUE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES,EN PARTICULIER LE DICHLORURE DE RADIUM 223; REGISTRATION NO/DATE: EU/1/13/873 20131113 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.