Teva Branded Pharm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA BRANDED PHARM, and what generic alternatives to TEVA BRANDED PHARM drugs are available?
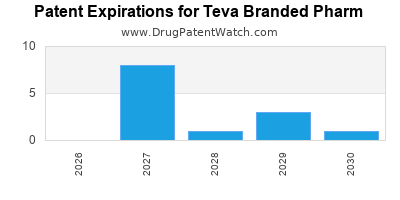
TEVA BRANDED PHARM has thirty-three approved drugs.
There are fifty-nine US patents protecting TEVA BRANDED PHARM drugs.
There are six hundred and eighty patent family members on TEVA BRANDED PHARM drugs in forty-one countries and thirty-nine supplementary protection certificates in fourteen countries.
Summary for Teva Branded Pharm
| International Patents: | 680 |
| US Patents: | 59 |
| Tradenames: | 33 |
| Ingredients: | 19 |
| NDAs: | 33 |
| Patent Litigation for Teva Branded Pharm: | See patent lawsuits for Teva Branded Pharm |
Drugs and US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | PROAIR RESPICLICK | albuterol sulfate | POWDER, METERED;INHALATION | 205636-001 | Mar 31, 2015 | RX | Yes | Yes | 9,216,260 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Teva Branded Pharm | PROAIR DIGIHALER | albuterol sulfate | POWDER, METERED;INHALATION | 205636-002 | Dec 21, 2018 | RX | Yes | No | 11,266,796 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Teva Branded Pharm | PROAIR RESPICLICK | albuterol sulfate | POWDER, METERED;INHALATION | 205636-001 | Mar 31, 2015 | RX | Yes | Yes | 10,124,131 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva Branded Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | QVAR 80 | beclomethasone dipropionate | AEROSOL, METERED;INHALATION | 020911-001 | Sep 15, 2000 | 5,776,432 | ⤷ Try a Trial |
| Teva Branded Pharm | LOXITANE | loxapine succinate | CAPSULE;ORAL | 017525-003 | Approved Prior to Jan 1, 1982 | 3,546,226 | ⤷ Try a Trial |
| Teva Branded Pharm | SEASONIQUE | ethinyl estradiol; levonorgestrel | TABLET;ORAL | 021840-001 | May 25, 2006 | 7,615,545 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TEVA BRANDED PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
International Patents for Teva Branded Pharm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Serbia | 58574 | ⤷ Try a Trial |
| Spain | 2561491 | ⤷ Try a Trial |
| European Patent Office | 3185938 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Branded Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | C20160011 00192 | Estonia | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREEL JA ETUENUEUELOESTRADIOOL;REG NO/DATE: EE 894715 11.11.2015 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| 1602370 | 2009/010 | Ireland | ⤷ Try a Trial | PRODUCT NAME: ALISKIREN OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF AND HYDROCHLOROTHIAZIDE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF.; NAT REGISTRATION NO/DATE: EU/1/08/491/001-EU/1/08/491/080 20090116; FIRST REGISTRATION NO/DATE: 58935 01 58935 02 58935 03 58935 04 20081028 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.