Teva Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA, and when can generic versions of TEVA drugs launch?
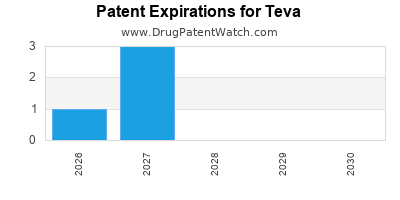
TEVA has seven hundred and thirty-six approved drugs.
There are one hundred US patents protecting TEVA drugs. There are fifty-three tentative approvals on TEVA drugs.
There are nine hundred and thirteen patent family members on TEVA drugs in forty-nine countries and one thousand and fifty-six supplementary protection certificates in eighteen countries.
Summary for Teva
| International Patents: | 913 |
| US Patents: | 100 |
| Tradenames: | 508 |
| Ingredients: | 443 |
| NDAs: | 736 |
| Patent Litigation for Teva: | See patent lawsuits for Teva |
| PTAB Cases with Teva as petitioner: | See PTAB cases with Teva as petitioner |
Drugs and US Patents for Teva
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Pharms Usa | CLARAVIS | isotretinoin | CAPSULE;ORAL | 076135-001 | Apr 11, 2003 | AB1 | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Pharms | MICONAZOLE NITRATE | miconazole nitrate | CREAM;VAGINAL | 074030-001 | Oct 30, 1992 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms Usa Inc | DROXIDOPA | droxidopa | CAPSULE;ORAL | 213162-002 | Feb 18, 2021 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms | METHYLPHENIDATE HYDROCHLORIDE | methylphenidate hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 077707-001 | Jul 19, 2012 | AB2 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva | ALBUTEROL SULFATE | albuterol sulfate | TABLET;ORAL | 072938-001 | Mar 30, 1990 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva | SILDENAFIL CITRATE | sildenafil citrate | TABLET;ORAL | 077342-001 | Mar 9, 2016 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Pharms | NADOLOL | nadolol | TABLET;ORAL | 074368-001 | Aug 31, 1994 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Teva Pharm | ARMONAIR RESPICLICK | fluticasone propionate | POWDER;INHALATION | 208798-002 | Jan 27, 2017 | 6,871,646 | ⤷ Try a Trial |
| Teva Branded Pharm | MIRCETTE | desogestrel; ethinyl estradiol | TABLET;ORAL-28 | 020713-001 | Apr 22, 1998 | 4,921,843 | ⤷ Try a Trial |
| Teva | AZILECT | rasagiline mesylate | TABLET;ORAL | 021641-002 | May 16, 2006 | 5,387,612 | ⤷ Try a Trial |
| Teva Parenteral | CYTARABINE | cytarabine | INJECTABLE;INJECTION | 016793-001 | Approved Prior to Jan 1, 1982 | 3,444,294 | ⤷ Try a Trial |
| Teva | AZILECT | rasagiline mesylate | TABLET;ORAL | 021641-001 | May 16, 2006 | 5,786,390 | ⤷ Try a Trial |
| Teva Branded Pharm | PROAIR DIGIHALER | albuterol sulfate | POWDER, METERED;INHALATION | 205636-002 | Dec 21, 2018 | 6,871,646 | ⤷ Try a Trial |
| Teva Pharms Usa | COPAXONE | glatiramer acetate | INJECTABLE;SUBCUTANEOUS | 020622-002 | Feb 12, 2002 | 6,342,476 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for TEVA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | for Injection | 3.5 mg/vial | ➤ Subscribe | 2016-10-26 |
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-05-17 |
| ➤ Subscribe | Extended-release Capsule | 15 mg and 30 mg | ➤ Subscribe | 2008-08-11 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-01-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg | ➤ Subscribe | 2004-03-29 |
| ➤ Subscribe | Tablets | 0.15 mg/0.02 mg, 0.15 mg/0.025 mg, 0.15 mg/0.03 mg and 0.01 mg | ➤ Subscribe | 2013-07-10 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2006-04-17 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 20 mg, 30 mg | ➤ Subscribe | 2009-11-18 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-02-26 |
| ➤ Subscribe | Tablets | 0.15 mg/0.03 mg/0.01 mg | ➤ Subscribe | 2008-01-22 |
| ➤ Subscribe | Tablets | 0.1 mg/0.02 mg and 0.01 mg | ➤ Subscribe | 2009-11-16 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
Premature patent expirations for TEVA
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Teva Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 102057 | ⤷ Try a Trial |
| Mexico | 2019010913 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005113044 | ⤷ Try a Trial |
| Brazil | 112013008824 | ⤷ Try a Trial |
| Japan | 2016025944 | ⤷ Try a Trial |
| Cyprus | 1114421 | ⤷ Try a Trial |
| South Korea | 20130103824 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0627406 | SPC/GB11/026 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: FINGOLIMOD, I.E. 2-AMINO-2-(2-(4-OCTYLPHENYL)ETHYL)PROPANE-1,3-DIOL, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/11/677/001 20110317; UK EU/1/11/677/002 20110317; UK EU/1/11/677/003 20110317; UK EU/1/11/677/004 20110317 |
| 2487163 | CA 2017 00003 | Denmark | ⤷ Try a Trial | PRODUCT NAME: COBICISTAT ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF OG ATAZANAVIR ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, ISAER ATAZANAVIRSULFAT; REG. NO/DATE: EU/1/15/1025/001-002 20150715 |
| 0720599 | SPC/GB05/010 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF IN COMBINATION WITH SIMVASTATIN; REGISTERED: DE 58874.00.00 20040402; DE 58874.01.00 20040402; DE 58874.02.00 20040402; DE 58874.03.00 20040402; DE 58878.00.00 20040402; DE 58878.01.00 20040402; DE 58878.02.00 20040402; DE 58878.03.00 20040402; DE 58866.00.00 20040402; DE 58866.01.00 20040402; DE 58866.02.00 20040402; DE 58866.03.00 20040402; DE 58870.00.00 20040402; DE 58870.01.00 20040402; DE 58870.02.00 20040402; DE 58870.03.00 20040402; UK PL 19945/0003 20041118; UK PL 19945/0004 20041118; UK PL 19945/0005 20041118; UK PL 19945/0006 20041118; UK PL 19945/0007 20041118; UK PL 19945/0008 20041118; UK PL 19945/0009 200411 |
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| 1663240 | SPC/GB15/064 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF RILPIVIRINE OR A PHARMACEUTICALLY ACCEPTABLE SALT OF RILPIVIRINE, INCLUDING THE HYDROCHLORIDE SALT OF RILPIVIRINE, AND EMTRICITABINE; REGISTERED: UK EU/1/11/737/001-002 20111128 |
| 0503785 | C300375 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN OLMESARTAN MEDOXOMIL, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT EN AMLODIPINEBESILAAT; REGISTRATION NO/DATE: RVG100984, RVG100986-87, RVG100989-91, RVG100993-95 20080819 |
| 1110543 | 08C0004 | France | ⤷ Try a Trial | PRODUCT NAME: DESLORATADINE; PSEUDOEPHEDRINE SULFATE; REGISTRATION NO/DATE: EU/1/07/399/001 20070730 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.