Salix Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SALIX PHARMS, and what generic alternatives to SALIX PHARMS drugs are available?
SALIX PHARMS has nine approved drugs.
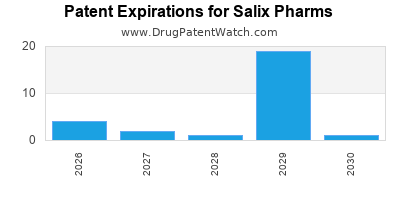
There are forty US patents protecting SALIX PHARMS drugs.
There are three hundred and twenty-three patent family members on SALIX PHARMS drugs in forty-three countries and eighty-six supplementary protection certificates in eleven countries.
Drugs and US Patents for Salix Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salix Pharms | MOVIPREP | ascorbic acid; polyethylene glycol 3350; potassium chloride; sodium ascorbate; sodium chloride; sodium sulfate | FOR SOLUTION;ORAL | 021881-001 | Aug 2, 2006 | AA | RX | Yes | Yes | 7,658,914 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-001 | May 25, 2004 | RX | Yes | Yes | 7,902,206 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Salix Pharms | PEPCID | famotidine | FOR SUSPENSION;ORAL | 019527-001 | Feb 2, 1987 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-001 | Apr 24, 2008 | RX | Yes | Yes | 9,492,445 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Salix Pharms | XIFAXAN | rifaximin | TABLET;ORAL | 021361-002 | Mar 24, 2010 | RX | Yes | Yes | 11,564,912 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Salix Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-001 | Apr 24, 2008 | 8,552,025 | ⤷ Try a Trial |
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-002 | Sep 27, 2010 | 6,559,158 | ⤷ Try a Trial |
| Salix Pharms | PEPCID | famotidine | FOR SUSPENSION;ORAL | 019527-001 | Feb 2, 1987 | 4,283,408*PED | ⤷ Try a Trial |
| Salix Pharms | METOZOLV ODT | metoclopramide hydrochloride | TABLET, ORALLY DISINTEGRATING;ORAL | 022246-001 | Sep 4, 2009 | 6,413,549 | ⤷ Try a Trial |
| Salix Pharms | RELISTOR | methylnaltrexone bromide | SOLUTION;SUBCUTANEOUS | 021964-003 | Sep 27, 2010 | 8,552,025 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SALIX PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 12 mg/0.6 mL | ➤ Subscribe | 2015-07-22 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2016-09-06 |
| ➤ Subscribe | Tablets | 1.102 g and 0.398 g | ➤ Subscribe | 2008-04-09 |
| ➤ Subscribe | Tablets | 200 mg | ➤ Subscribe | 2019-01-28 |
| ➤ Subscribe | For Oral Solution | 100 g, 7.5 g, 2.691 g, 1.015 g, 5.9 g and 4.7 g per pouch | ➤ Subscribe | 2007-11-27 |
| ➤ Subscribe | Injection | 8 mg/0.4 mL | ➤ Subscribe | 2015-09-08 |
| ➤ Subscribe | Orally Disintegrating Tablets | 5 mg and 10 mg | ➤ Subscribe | 2010-08-24 |
| ➤ Subscribe | Tablets | 550 mg | ➤ Subscribe | 2015-12-18 |
International Patents for Salix Pharms Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2020196733 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2011005388 | ⤷ Try a Trial |
| Lithuania | 3628319 | ⤷ Try a Trial |
| Spain | 2320160 | ⤷ Try a Trial |
| Poland | 3608322 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Salix Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1758590 | 2017C/063 | Belgium | ⤷ Try a Trial | PRODUCT NAME: SEL DE SODIUM D'ACIDE DESOXYCHOLIQUE; AUTHORISATION NUMBER AND DATE: SE/H/1547/01/DC 20170612 |
| 1713823 | 1490064-1 | Sweden | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR, OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, INCLUDING SIMEPREVIR SODIUM; REG. NO/DATE: EU/1/14/924 20140516 |
| 1175904 | 2007C/048 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ALENDRONATE DE SODIUM/COLECALCIFEROL; AUTHORISATION NUMBER AND DATE: EU/1/05/310/001 20050826 |
| 0145340 | 99C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: FOSPHENYTOIN DISODIUM; NAT. REGISTRATION NO/DATE: NL 23 613 19980806; FIRST REGISTRATION: GB - PL 000 19/0157 19980204 |
| 0806968 | SPC/GB07/011 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: GADOFOSVESET TRISODIUM; REGISTERED: UK EU/1/05/313/001 20051003; UK EU/1/05/313/002 20051003; UK EU/1/05/313/003 20051003; UK EU/1/05/313/004 20051003; UK EU/1/05/313/005 20051003; UK EU/1/05/313/006 20051003; UK EU/1/05/313/007 20051003; UK EU/1/05/313/008 20051003; UK EU/1/05/313/009 20051003 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.