Novo Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVO, and when can generic versions of NOVO drugs launch?
NOVO has eleven approved drugs.
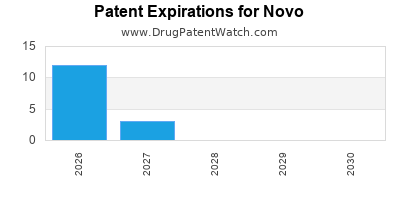
There are fifty-six US patents protecting NOVO drugs.
There are four hundred and sixteen patent family members on NOVO drugs in forty countries and one hundred and eighteen supplementary protection certificates in seventeen countries.
Summary for Novo
| International Patents: | 416 |
| US Patents: | 56 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 11 |
| Patent Litigation for Novo: | See patent lawsuits for Novo |
Drugs and US Patents for Novo
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | RX | Yes | Yes | 9,132,239 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | WEGOVY | semaglutide | SOLUTION;SUBCUTANEOUS | 215256-003 | Jun 4, 2021 | RX | Yes | Yes | 8,129,343 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | 9,265,893*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Novo
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | PRANDIMET | metformin hydrochloride; repaglinide | TABLET;ORAL | 022386-002 | Jun 23, 2008 | 6,677,358 | ⤷ Try a Trial |
| Novo Nordisk Inc | VICTOZA | liraglutide | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RE41956*PED | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-003 | Mar 28, 2022 | 8,579,869 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVO drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 18 mg/3 mL prefilled syringe | ➤ Subscribe | 2016-12-12 |
| ➤ Subscribe | Vaginal Tablets | 10 mcg | ➤ Subscribe | 2013-01-02 |
| ➤ Subscribe | Tablets | 1 mg/500 mg and 2 mg/500 mg | ➤ Subscribe | 2009-04-09 |
International Patents for Novo Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2004290862 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2021144476 | ⤷ Try a Trial |
| Denmark | 3423082 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Novo Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | C01261586/02 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN UND METFORMIN; NAT. REGISTRATION NO/DATE: SWISSMEDIC 62040 20120329 |
| 1261586 | 2012C/016 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE PRODUITS DE SAXAGLIPTINE ET DE METFORMINE AINSI QUE TOUT SELS PHARMACEUTIQUEMENT ACCEPTABLES, DONT LES SELS DE CHLORHYDRATE DE SAXAGLIPTINE ET DE METFORMINE; AUTHORISATION NUMBER AND DATE: EU/1/11/731/001 20111128 |
| 1412357 | PA2008013,C1412357 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINUM PHOSPHAS MONOHYDRICUS, METFORMINI HYDROCHLORIDUM; REGISTRATION NO/DATE: EU/1/08/455/001 - EU/1/08/455/014 20080716 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.