Noven Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVEN, and what generic alternatives to NOVEN drugs are available?
NOVEN has eight approved drugs.
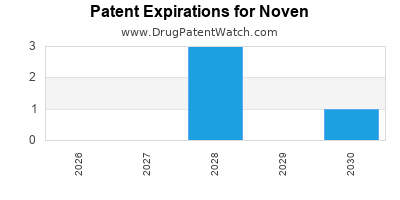
There are eleven US patents protecting NOVEN drugs. There are three tentative approvals on NOVEN drugs.
There are fifty-four patent family members on NOVEN drugs in eighteen countries and fifty-two supplementary protection certificates in fourteen countries.
Summary for Noven
| International Patents: | 54 |
| US Patents: | 11 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 8 |
| Patent Litigation for Noven: | See patent lawsuits for Noven |
| PTAB Cases with Noven as petitioner: | See PTAB cases with Noven as petitioner |
Drugs and US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-002 | Mar 22, 2022 | RX | Yes | No | 8,591,941 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-003 | Apr 6, 2006 | AB | RX | Yes | No | 9,668,981 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-001 | Mar 22, 2022 | RX | Yes | No | 9,474,722 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven | FENTANYL-25 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 077775-001 | Oct 16, 2009 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-004 | Mar 22, 2022 | RX | Yes | Yes | 9,034,370 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-004 | Apr 6, 2006 | AB | RX | Yes | Yes | 9,034,370 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-003 | Apr 6, 2006 | AB | RX | Yes | No | 8,632,802 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-001 | Apr 6, 2006 | 5,958,446 | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-004 | Oct 29, 2012 | 6,024,976 | ⤷ Try a Trial |
| Noven Pharms Inc | COMBIPATCH | estradiol; norethindrone acetate | FILM, EXTENDED RELEASE;TRANSDERMAL | 020870-001 | Aug 7, 1998 | 5,656,286 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-003 | Apr 6, 2006 | 6,210,705 | ⤷ Try a Trial |
| Noven | DENTIPATCH | lidocaine | FILM, EXTENDED RELEASE;BUCCAL | 020575-001 | May 21, 1996 | 5,446,070 | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-002 | Oct 29, 2012 | 5,656,286 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-004 | Apr 6, 2006 | 5,958,446 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVEN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Transdermal System | 0.0375 mg/day, 0.05 mg/day, 0.075 mg/day, 0.1 mg/day | ➤ Subscribe | 2014-08-18 |
| ➤ Subscribe | Transdermal System | 10 mg/9 hrs, 15 mg/9 hrs, 20 mg/9 hrs and 30 mg/9 hrs | ➤ Subscribe | 2011-04-13 |
| ➤ Subscribe | Transdermal System | 0.025 mg/day | ➤ Subscribe | 2015-05-08 |
International Patents for Noven Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2010006143 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006044206 | ⤷ Try a Trial |
| China | 116997330 | ⤷ Try a Trial |
| Mexico | 2016008207 | ⤷ Try a Trial |
| European Patent Office | 1807033 | ⤷ Try a Trial |
| European Patent Office | 2934496 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2014066585 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Noven Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 0398460 | 12/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON IN KOMBINATION MIT ESTRADIOL; NAT. REGISTRATION NO/DATE: 1-25178, 1-25179 20031127; FIRST REGISTRATION: NL RVG 27505 20021211 |
| 1453521 | C201630040 | Spain | ⤷ Try a Trial | PRODUCT NAME: ETINILESTRADIOL Y MEZCLA DE LEVONORGESTREL Y ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: 80340; DATE OF AUTHORISATION: 20160122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150211 |
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| 1453521 | 39/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1453521 | CA 2016 00016 | Denmark | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL OG ETHINYLOESTRADIOL; NAT. REG. NO/DATE: 56336 20151105; FIRST REG. NO/DATE: SK 17/0017/15-S 20150211 |
| 0398460 | SPC/GB04/032 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL, OPTIONALLY IN THE FORM OF A HYDRATE, TOGETHER WITH DROSPIRENONE; REGISTERED: NL RVG 27505 20021211; UK PL 00053/0341 20040310 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.