Emd Serono Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for EMD SERONO INC, and what generic alternatives to EMD SERONO INC drugs are available?
EMD SERONO INC has six approved drugs.
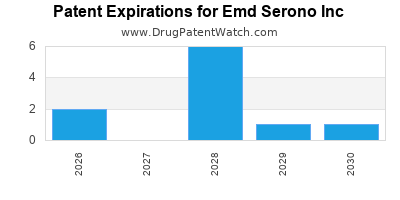
There are thirteen US patents protecting EMD SERONO INC drugs.
There are one hundred and ninety-five patent family members on EMD SERONO INC drugs in forty-two countries and eighty-nine supplementary protection certificates in sixteen countries.
Summary for Emd Serono Inc
| International Patents: | 195 |
| US Patents: | 13 |
| Tradenames: | 6 |
| Ingredients: | 5 |
| NDAs: | 6 |
| Drug Master File Entries: | 2 |
Drugs and US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-001 | Sep 26, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | MAVENCLAD | cladribine | TABLET;ORAL | 022561-001 | Mar 29, 2019 | RX | Yes | Yes | 7,888,328 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | 8,580,781 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | 7,605,121 | ⤷ Try a Trial |
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | 6,319,192 | ⤷ Try a Trial |
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-002 | Sep 26, 1997 | 4,517,181 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Emd Serono Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2016138128 | ⤷ Try a Trial |
| European Patent Office | 4070800 | ⤷ Try a Trial |
| Slovenia | 1608344 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Emd Serono Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2498758 | 2020C/509 | Belgium | ⤷ Try a Trial | PRODUCT NAME: QTRILMET - METFORMINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; SAXAGLIPTINE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; DAPAGLIFLOZINE OU UN SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI; AUTHORISATION NUMBER AND DATE: EU/1/19/1401 20191113 |
| 2805723 | C20180006 00238 | Estonia | ⤷ Try a Trial | PRODUCT NAME: KLADRIBIIN;REG NO/DATE: EU/1/17/1212 24.08.2017 |
| 1608344 | 372 4-2018 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: KLADRIBIN; REGISTRATION NO/DATE: EU/1/17/1212 20170824 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.