Emd Serono Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for EMD SERONO INC, and when can generic versions of EMD SERONO INC drugs launch?
EMD SERONO INC has six approved drugs.
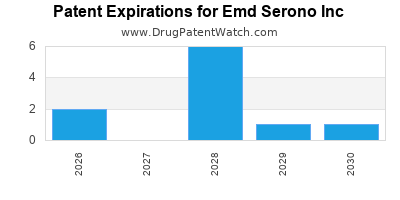
There are thirteen US patents protecting EMD SERONO INC drugs.
There are one hundred and ninety-five patent family members on EMD SERONO INC drugs in forty-two countries and eighty-nine supplementary protection certificates in sixteen countries.
Summary for Emd Serono Inc
| International Patents: | 195 |
| US Patents: | 13 |
| Tradenames: | 6 |
| Ingredients: | 5 |
| NDAs: | 6 |
| Drug Master File Entries: | 2 |
Drugs and US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Emd Serono Inc | GLUCOPHAGE | metformin hydrochloride | TABLET;ORAL | 020357-004 | Nov 5, 1998 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | 9,403,799 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Emd Serono Inc | TEPMETKO | tepotinib hydrochloride | TABLET;ORAL | 214096-001 | Feb 3, 2021 | RX | Yes | Yes | 8,927,540 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Emd Serono Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-002 | Aug 11, 2000 | 6,863,891 | ⤷ Try a Trial |
| Emd Serono Inc | CETROTIDE | cetrorelix acetate | POWDER;SUBCUTANEOUS | 021197-001 | Aug 11, 2000 | 4,800,191 | ⤷ Try a Trial |
| Emd Serono Inc | GLUCOPHAGE XR | metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021202-001 | Oct 13, 2000 | 6,475,521 | ⤷ Try a Trial |
| Emd Serono Inc | GEREF | sermorelin acetate | INJECTABLE;INJECTION | 020443-002 | Sep 26, 1997 | 4,517,181 | ⤷ Try a Trial |
| Emd Serono Inc | GLUCOPHAGE XR | metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021202-001 | Oct 13, 2000 | 6,660,300 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Emd Serono Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 5412709 | ⤷ Try a Trial |
| Argentina | 067505 | ⤷ Try a Trial |
| Denmark | 2272503 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2009006959 | ⤷ Try a Trial |
| Denmark | 3332789 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Emd Serono Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1506211 | C01506211/02 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZIN + METFORMIN; REGISTRATION NO/DATE: SWISSMEDIC 65377 16.07.2015 |
| 1412357 | C300357 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVA ARDBAAR ZOUT, IN HET BIJZONDER HET MONOFOSFAAT, EN METFORMINE DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/08/455/001-014 20080716 |
| 0299402 | 099C0031 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ACETATE DE CETRORELIX; REGISTRATION NO/DATE: EU/1/99/100/001 19990413 |
| 1608344 | C 2018 009 | Romania | ⤷ Try a Trial | PRODUCT NAME: CLADRIBINA; NATIONAL AUTHORISATION NUMBER: EU/1/17/1212; DATE OF NATIONAL AUTHORISATION: 20170822; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1212; DATE OF FIRST AUTHORISATION IN EEA: 20170822 |
| 1261586 | 1290013-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: SAXAGLIPTIN/METFORMIN; REG. NO/DATE: EU/1/11/731/001 20111124 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.