Bristol Myers Squibb Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BRISTOL MYERS SQUIBB, and what generic alternatives to BRISTOL MYERS SQUIBB drugs are available?
BRISTOL MYERS SQUIBB has seventy approved drugs.
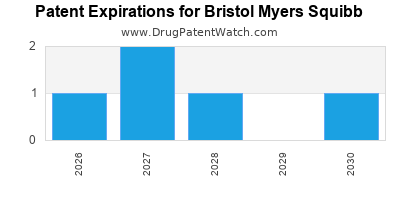
There are thirteen US patents protecting BRISTOL MYERS SQUIBB drugs.
There are five hundred and ninety-six patent family members on BRISTOL MYERS SQUIBB drugs in fifty-one countries and one hundred and forty-three supplementary protection certificates in eighteen countries.
Summary for Bristol Myers Squibb
| International Patents: | 596 |
| US Patents: | 13 |
| Tradenames: | 60 |
| Ingredients: | 55 |
| NDAs: | 70 |
Drugs and US Patents for Bristol Myers Squibb
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bristol Myers Squibb | ELIQUIS | apixaban | TABLET;ORAL | 202155-001 | Dec 28, 2012 | AB | RX | Yes | No | 9,326,945 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Bristol Myers Squibb | MONOPRIL | fosinopril sodium | TABLET;ORAL | 019915-003 | May 16, 1991 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bristol Myers Squibb | AMNESTROGEN | estrogens, esterified | TABLET;ORAL | 083266-003 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-003 | Jun 29, 2006 | AB | RX | Yes | No | 7,855,217 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Bristol Myers Squibb | BUSPAR | buspirone hydrochloride | CAPSULE;ORAL | 021190-002 | Dec 20, 2000 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bristol Myers Squibb | VIDEX EC | didanosine | CAPSULE, DELAYED REL PELLETS;ORAL | 021183-003 | Oct 31, 2000 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bristol Myers Squibb
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bristol Myers Squibb | VIDEX | didanosine | TABLET, CHEWABLE;ORAL | 020154-005 | Oct 9, 1991 | 4,861,759*PED | ⤷ Try a Trial |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-004 | Jun 29, 2006 | 5,635,517 | ⤷ Try a Trial |
| Bristol Myers Squibb | ZERIT XR | stavudine | CAPSULE, EXTENDED RELEASE;ORAL | 021453-003 | Dec 31, 2002 | 4,978,655*PED | ⤷ Try a Trial |
| Bristol Myers Squibb | SERZONE | nefazodone hydrochloride | TABLET;ORAL | 020152-001 | Dec 22, 1994 | 5,256,664*PED | ⤷ Try a Trial |
| Bristol Myers Squibb | REVLIMID | lenalidomide | CAPSULE;ORAL | 021880-001 | Dec 27, 2005 | 7,189,740 | ⤷ Try a Trial |
| Bristol Myers Squibb | REYATAZ | atazanavir sulfate | CAPSULE;ORAL | 021567-003 | Jun 20, 2003 | 5,849,911*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BRISTOL MYERS SQUIBB drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 80 mg and 140 mg | ➤ Subscribe | 2011-06-16 |
| ➤ Subscribe | Capsules | 25 mg | ➤ Subscribe | 2010-07-12 |
| ➤ Subscribe | Capsules | 2.5 mg and 20 mg | ➤ Subscribe | 2016-07-12 |
| ➤ Subscribe | Capsules | 100 mg and 150 mg | ➤ Subscribe | 2010-03-19 |
| ➤ Subscribe | Tablets | 600 mg | ➤ Subscribe | 2009-04-09 |
| ➤ Subscribe | Nasal Spray | 4 mg/spray | ➤ Subscribe | 2016-07-15 |
| ➤ Subscribe | Tablets | 2.5 mg and 5 mg | ➤ Subscribe | 2016-12-28 |
| ➤ Subscribe | Tablets | 20 mg, 50 mg, 70 mg and 100 mg | ➤ Subscribe | 2010-06-28 |
| ➤ Subscribe | Tablets | 80 mg and 140 mg | ➤ Subscribe | 2011-06-17 |
| ➤ Subscribe | Delayed-release Capsules | 200 mg, 250 mg and 400 mg | ➤ Subscribe | 2004-06-01 |
| ➤ Subscribe | Capsules | 5 mg, 10 mg and 15 mg | ➤ Subscribe | 2010-08-30 |
| ➤ Subscribe | Capsules | 300 mg | ➤ Subscribe | 2009-07-20 |
| ➤ Subscribe | Capsules | 200 mg | ➤ Subscribe | 2010-02-16 |
| ➤ Subscribe | Capsules | 50 mg, 100 mg and 200 mg | ➤ Subscribe | 2016-11-03 |
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-06-14 |
| ➤ Subscribe | Tablets | 30 mg | ➤ Subscribe | 2005-06-01 |
International Patents for Bristol Myers Squibb Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Peru | 20130378 | ⤷ Try a Trial |
| China | 103495169 | ⤷ Try a Trial |
| Japan | 2008519049 | ⤷ Try a Trial |
| Canada | 2752124 | ⤷ Try a Trial |
| Japan | 2009191072 | ⤷ Try a Trial |
| Chile | 2008002717 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bristol Myers Squibb Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1427415 | C 2011 008 | Romania | ⤷ Try a Trial | PRODUCT NAME: APIXABANSI SARURI ACCEPTABILE FARMACEUTIC ALE ACESTUIA; NATIONAL AUTHORISATION NUMBER: RO EU/1/11/691/001, RO EU/1/11/691/002, RO EU/1/11/691/003, RO EU/1/11/691/004, RO EU/1/11/691/005; DATE OF NATIONAL AUTHORISATION: 20110518; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/11/691/001, EU/1/11/691/002, EU/1/11/691/003, EU/1/11/691/004, EU/1/11/691/005; DATE OF FIRST AUTHORISATION IN EEA: 20110518 |
| 1685839 | 92292 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON D OXYCODONE EN TANT QUE COMPOSANT A ET DE NALOXONE EN TANT QUE COMPOSANT B SOUS TOUTES LES FORMES PROTEGES PAR LE BREVET DE BASE |
| 0216510 | 94C0006 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DIDANOSINE; NAT. REG.: 203 IS 225 F 3 19930331; FIRST REG.: FR 557 386-4 19920505 |
| 0136011 | C00136011/03 | Switzerland | ⤷ Try a Trial | PRODUCT NMAE: ESTRADIOL UND MEDROXYPROGESTERONACETAT; REGISTRATION NO/DATE: IKS 55288 20000417 |
| 1453521 | 93156 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ET ETHINYLESTRADIOL; FIRST REGISTRATION DATE: 20150211 |
| 2487163 | PA2016039 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ATAZANAVIRAS + KOBICISTATAS; REGISTRATION NO/DATE: EU/1/15/1025 20150713 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.