Bausch Company Profile
✉ Email this page to a colleague
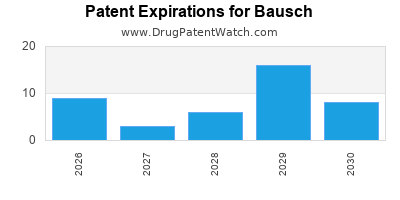
What is the competitive landscape for BAUSCH, and when can generic versions of BAUSCH drugs launch?
BAUSCH has one hundred and seventy-six approved drugs.
There are one hundred and twenty-one US patents protecting BAUSCH drugs.
There are seven hundred and seventy-five patent family members on BAUSCH drugs in fifty-two countries and two hundred and eight supplementary protection certificates in sixteen countries.
Summary for Bausch
| International Patents: | 775 |
| US Patents: | 121 |
| Tradenames: | 155 |
| Ingredients: | 122 |
| NDAs: | 176 |
| Patent Litigation for Bausch: | See patent lawsuits for Bausch |
Drugs and US Patents for Bausch
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | OLOPATADINE HYDROCHLORIDE | olopatadine hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 206046-001 | Jul 26, 2017 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bausch Lomb Ireland | FLUORESCEIN SODIUM AND BENOXINATE HYDROCHLORIDE | benoxinate hydrochloride; fluorescein sodium | SOLUTION/DROPS;OPHTHALMIC | 211039-001 | Mar 9, 2020 | RX | Yes | Yes | 10,842,872 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bausch | DUOBRII | halobetasol propionate; tazarotene | LOTION;TOPICAL | 209354-001 | Apr 25, 2019 | RX | Yes | Yes | 10,478,502 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bausch | CABTREO | adapalene; benzoyl peroxide; clindamycin phosphate | GEL;TOPICAL | 216632-001 | Oct 20, 2023 | RX | Yes | Yes | 10,220,049 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bausch
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | VANOS | fluocinonide | CREAM;TOPICAL | 021758-001 | Feb 11, 2005 | 7,220,424 | ⤷ Try a Trial |
| Bausch | CARDIZEM LA | diltiazem hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021392-005 | Feb 6, 2003 | 6,923,984 | ⤷ Try a Trial |
| Bausch | CARDIZEM LA | diltiazem hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021392-005 | Feb 6, 2003 | 7,108,866 | ⤷ Try a Trial |
| Bausch | SOLODYN | minocycline hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 050808-004 | Jul 23, 2009 | 5,908,838 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAUSCH drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Gel | 0.77% | ➤ Subscribe | 2006-05-10 |
| ➤ Subscribe | Lotion | 0.1% | ➤ Subscribe | 2016-08-31 |
| ➤ Subscribe | Extended-release Tablets | 174 mg | ➤ Subscribe | 2009-09-28 |
| ➤ Subscribe | Cream | 3.75% | ➤ Subscribe | 2012-08-08 |
| ➤ Subscribe | Extended-release Tablets | 522 mg | ➤ Subscribe | 2009-12-24 |
| ➤ Subscribe | For Inhalation Solution | 6 gm/vial | ➤ Subscribe | 2014-05-22 |
| ➤ Subscribe | Extended-release Tablets | 420 mg | ➤ Subscribe | 2005-04-25 |
| ➤ Subscribe | Rectal Gel | 5 mg/mL, 4mL pre-filled syringe | ➤ Subscribe | 2008-12-08 |
| ➤ Subscribe | Extended-release Tablet | 55 mg and 80 mg | ➤ Subscribe | 2010-12-02 |
| ➤ Subscribe | Gel | 1.2%/2.5% | ➤ Subscribe | 2012-12-20 |
| ➤ Subscribe | Extended-release Tablets | 150 mg and 300 mg | ➤ Subscribe | 2004-09-21 |
| ➤ Subscribe | Gel | 0.1% | ➤ Subscribe | 2010-07-08 |
| ➤ Subscribe | Ophthalmic Solution | 0.07% | ➤ Subscribe | 2013-07-26 |
| ➤ Subscribe | Capsules | 75 mg | ➤ Subscribe | 2011-06-06 |
| ➤ Subscribe | Vaginal Gel | 0.75% | ➤ Subscribe | 2004-09-02 |
| ➤ Subscribe | Cream | 5% | ➤ Subscribe | 2006-10-17 |
| ➤ Subscribe | Topical Solution | 10% | ➤ Subscribe | 2018-06-06 |
| ➤ Subscribe | Cream | 0.10% | ➤ Subscribe | 2008-01-31 |
| ➤ Subscribe | Extended-release Tablets | 348 mg | ➤ Subscribe | 2009-09-24 |
| ➤ Subscribe | Cream | 2.5% | ➤ Subscribe | 2014-06-17 |
| ➤ Subscribe | Extended-release Tablets | 120 mg, 180 mg, 240 mg, 300 mg and 360 mg | ➤ Subscribe | 2005-08-30 |
| ➤ Subscribe | Rectal Gel | 2.5 mg/0.5 mL, 5 mg/mL, 10 mg/2 mL, 15 mg/3 mL and 20 mg/4 mL | ➤ Subscribe | 2004-03-23 |
| ➤ Subscribe | Extended-release Tablets | 65 mg and 115 mg | ➤ Subscribe | 2009-11-19 |
| ➤ Subscribe | Rectal Gel | 5 mg/mL, 2mL pre-filled syringe | ➤ Subscribe | 2008-12-23 |
| ➤ Subscribe | Extended-release Tablet | 105 rng | ➤ Subscribe | 2010-12-28 |
| ➤ Subscribe | Gel | 1.2%/3.75% | ➤ Subscribe | 2015-09-30 |
| ➤ Subscribe | Ophthalmic Solution | 0.5% | ➤ Subscribe | 2012-10-19 |
| ➤ Subscribe | Gel | 0.04% | ➤ Subscribe | 2010-12-20 |
| ➤ Subscribe | Ophthalmic Solution | 1.5% | ➤ Subscribe | 2013-09-09 |
| ➤ Subscribe | Gel | 1.2%/0.025% | ➤ Subscribe | 2010-12-17 |
International Patents for Bausch Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2890471 | ⤷ Try a Trial |
| Canada | 2894170 | ⤷ Try a Trial |
| Japan | 2015114210 | ⤷ Try a Trial |
| European Patent Office | 3495023 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bausch Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0427680 | SZ 12/2003 | Austria | ⤷ Try a Trial | PRODUCT NAME: PIMECROLIMUS, GEGEBENENFALLS IN FORM PHARMAZEUTISCH ANNEHMBARER SALZE |
| 0656775 | 28/2000 | Austria | ⤷ Try a Trial | PRODUCT NAME: BUPROPION HYDROCHLORID; NAT. REGISTRATION NO/DATE: 1-23680 20000616; FIRST REGISTRATION: NL 24160 19991201 |
| 0186405 | SPC/GB00/021 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: (1-HYDROXY-2-(3-PYRIDINYL)ETHYLIDENE)BIS(PHOSPHONIC ACID) "RESIDRONATE" AND SALTS THEREOF, ESPECIALLY THE SODIUM SALT; REGISTERED: SE 15296 19991007; SE 15297 19991007; UK PL 00364/0070 20000316 |
| 0427680 | 12/2003 | Austria | ⤷ Try a Trial | PRODUCT NAME: PIMECROLIMUS, GEGEBENENFALLS IN FORM PHARMAZEUTISCH ANNEHMBARER SALZE; NAT. REGISTRATION NO/DATE: 1-24689 20020917; FIRST REGISTRATION: DK 21034 20020315 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.