Haleon Us Holdings Company Profile
✉ Email this page to a colleague
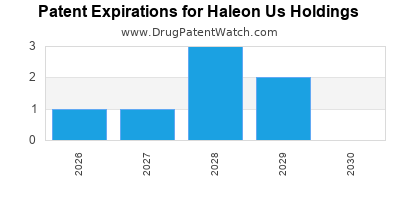
What is the competitive landscape for HALEON US HOLDINGS, and when can generic versions of HALEON US HOLDINGS drugs launch?
HALEON US HOLDINGS has thirty-one approved drugs.
There are ten US patents protecting HALEON US HOLDINGS drugs.
There are two hundred and three patent family members on HALEON US HOLDINGS drugs in thirty-two countries and sixteen supplementary protection certificates in eight countries.
Summary for Haleon Us Holdings
| International Patents: | 203 |
| US Patents: | 10 |
| Tradenames: | 28 |
| Ingredients: | 18 |
| NDAs: | 31 |
Drugs and US Patents for Haleon Us Holdings
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haleon Us Holdings | NICORETTE | nicotine polacrilex | TROCHE/LOZENGE;ORAL | 022360-001 | May 18, 2009 | OTC | Yes | No | 8,501,164 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Haleon Us Holdings | TAVIST-1 | clemastine fumarate | TABLET;ORAL | 020925-001 | Aug 21, 1992 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Haleon Us Holdings | ADVIL MIGRAINE LIQUI-GELS | ibuprofen | CAPSULE;ORAL | 020402-002 | Mar 16, 2000 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Haleon Us Holdings
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Haleon Us Holdings | FLONASE | fluticasone propionate | SPRAY, METERED;NASAL | 020121-001 | Oct 19, 1994 | 4,335,121*PED | ⤷ Try a Trial |
| Haleon Us Holdings | ADVIL PM | diphenhydramine citrate; ibuprofen | TABLET;ORAL | 021394-001 | Dec 21, 2005 | 8,263,647 | ⤷ Try a Trial |
| Haleon Us Holdings | ADVIL PM | diphenhydramine hydrochloride; ibuprofen | CAPSULE;ORAL | 021393-001 | Dec 21, 2005 | 8,883,849 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for HALEON US HOLDINGS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 200 mg/30 mg | ➤ Subscribe | 2004-12-27 |
| ➤ Subscribe | Capsules | 60 mg | ➤ Subscribe | 2010-09-08 |
| ➤ Subscribe | Gum | 4 mg | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Capsules | 200 mg/38 mg | ➤ Subscribe | 2016-02-16 |
| ➤ Subscribe | Gum | 2 mg | ➤ Subscribe | 2013-01-22 |
International Patents for Haleon Us Holdings Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2006235671 | ⤷ Try a Trial |
| Peru | 20081072 | ⤷ Try a Trial |
| European Patent Office | 1755561 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Haleon Us Holdings Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1305329 | 08C0014 | France | ⤷ Try a Trial | PRODUCT NAME: FLUTICASONE FUROATE; REGISTRATION NO/DATE: EU/1/07/434/001 20080111 |
| 2506844 | 132018000000341 | Italy | ⤷ Try a Trial | PRODUCT NAME: UN PRODOTTO DI COMBINAZIONE FARMACEUTICA COMPRENDENTE UN SALE FARMACEUTICAMENTE ACCETTABILE DI UMECLIDINIO (AD ESEMPIO BROMURO DI UMECLIDINIO), VILANTEROLO O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE (AD ESEMPIO VILANTEROLO TRIFENATATO) E UN FUROATO(TRELEGY ELLIPTA - FLUTICASONE FUROATO/UMECLIDINIO/VILANTEROLO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/17/1236, 20171117 |
| 1519731 | 132013902182575 | Italy | ⤷ Try a Trial | PRODUCT NAME: AZELASTINA CLORIDRATO/FLUTICASONE PROPIONATO(DYMISTA); AUTHORISATION NUMBER(S) AND DATE(S): 2011/07125-REG, 20111024;041808015/M-027/M-039/M-041/M, 20130527 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.