Aripiprazole - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for aripiprazole and what is the scope of freedom to operate?
Aripiprazole
is the generic ingredient in seven branded drugs marketed by Otsuka Pharm Co Ltd, Otsuka, Amneal Pharms, Apotex, Aurobindo Pharma Ltd, Chartwell Rx, Hetero Labs Ltd Iii, Lannett Co Inc, Rubicon, Vistapharm, Alembic, Orbion Pharms, Sciegen Pharms Inc, Square Pharms, Accord Hlthcare, Ajanta Pharma Ltd, Alkem Labs Ltd, Aurobindo Pharma, Breckenridge, Hetero Labs Ltd V, Macleods Pharms Ltd, Mylan, Prinston Inc, Sunshine, Teva Pharms Usa, Torrent, Unichem, Zydus Pharms, and Alkermes Inc, and is included in forty-two NDAs. There are sixty-two patents protecting this compound and three Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Aripiprazole has eight hundred and ninety-nine patent family members in forty-eight countries.
There are forty-nine drug master file entries for aripiprazole. Forty-seven suppliers are listed for this compound. There are five tentative approvals for this compound.
Summary for aripiprazole
| International Patents: | 899 |
| US Patents: | 62 |
| Tradenames: | 7 |
| Applicants: | 29 |
| NDAs: | 42 |
| Drug Master File Entries: | 49 |
| Finished Product Suppliers / Packagers: | 47 |
| Raw Ingredient (Bulk) Api Vendors: | 161 |
| Clinical Trials: | 384 |
| Patent Applications: | 7,090 |
| Formulation / Manufacturing: | see details |
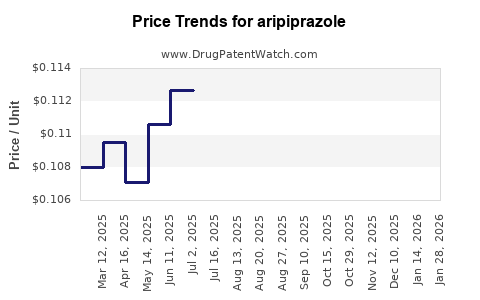
| Drug Prices: | Drug price trends for aripiprazole |
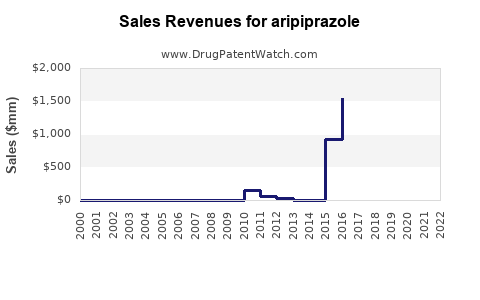
| Drug Sales Revenues: | Drug sales revenues for aripiprazole |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for aripiprazole |
| What excipients (inactive ingredients) are in aripiprazole? | aripiprazole excipients list |
| DailyMed Link: | aripiprazole at DailyMed |
Recent Clinical Trials for aripiprazole
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| All India Institute of Medical Sciences, Bhubaneswar | N/A |
| First Affiliated Hospital Xi'an Jiaotong University | N/A |
| First Affiliated Hospital of Chongqing Medical University | Phase 1/Phase 2 |
Generic filers with tentative approvals for ARIPIPRAZOLE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 30MG | TABLET; ORALLY DISINTEGRATING |
| ⤷ Try a Trial | ⤷ Try a Trial | 20MG | TABLET, ORALLY DISINTEGRATING; ORAL |
| ⤷ Try a Trial | ⤷ Try a Trial | 15MG | TABLET, ORALLY DISINTEGRATING; ORAL |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for aripiprazole
| Drug Class | Atypical Antipsychotic |
Medical Subject Heading (MeSH) Categories for aripiprazole
Anatomical Therapeutic Chemical (ATC) Classes for aripiprazole
Paragraph IV (Patent) Challenges for ARIPIPRAZOLE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ABILIFY MAINTENA KIT | Extended-release Injectable Suspension | aripiprazole | 300 mg/vial and 400 mg/vial | 202971 | 1 | 2021-12-20 |
| ABILIFY | Oral Solution | aripiprazole | 1 mg/mL | 021713 | 1 | 2007-12-20 |
| ABILIFY | Tablets | aripiprazole | 2 mg, 5 mg, 10 mg, 15 mg, 20 mg and 30 mg | 021436 | 8 | 2006-11-15 |
| ABILIFY | Orally Disintegrating Tablets | aripiprazole | 10 mg, 15 mg, 20 mg and 30 mg | 021729 | 1 | 2006-11-15 |
US Patents and Regulatory Information for aripiprazole
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apotex | ARIPIPRAZOLE | aripiprazole | TABLET;ORAL | 078583-002 | Jul 24, 2015 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-004 | Nov 13, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-005 | Nov 13, 2017 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-002 | Feb 28, 2013 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for aripiprazole
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Otsuka | ABILIFY | aripiprazole | TABLET, ORALLY DISINTEGRATING;ORAL | 021729-002 | Jun 7, 2006 | ⤷ Try a Trial | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET;ORAL | 021436-002 | Nov 15, 2002 | ⤷ Try a Trial | ⤷ Try a Trial |
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-004 | Sep 29, 2014 | ⤷ Try a Trial | ⤷ Try a Trial |
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-002 | Feb 28, 2013 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for aripiprazole
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Mylan Pharmaceuticals Limited | Aripiprazole Mylan Pharma (previously Aripiprazole Pharmathen) | aripiprazole | EMEA/H/C/003803 Aripiprazole Mylan Pharma is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.Aripiprazole Mylan Pharma is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.Aripiprazole Mylan Pharma is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older. |
Authorised | yes | no | no | 2015-06-30 | |
| Otsuka Pharmaceutical Netherlands B.V. | Abilify | aripiprazole | EMEA/H/C/000471 Abilify is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.Abilify is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.Abilify is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older. |
Authorised | no | no | no | 2004-06-04 | |
| Otsuka Pharmaceutical Netherlands B.V. | Abilify Maintena | aripiprazole | EMEA/H/C/002755 Maintenance treatment of schizophrenia in adult patients stabilised with oral aripiprazole. |
Authorised | no | no | no | 2013-11-14 | |
| Accord Healthcare S.L.U. | Aripiprazole Accord | aripiprazole | EMEA/H/C/004021 Aripiprazole Accord is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older., , Aripiprazole Accord is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment., , Aripiprazole Accord is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older., |
Authorised | yes | no | no | 2015-11-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for aripiprazole
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 2012284125 | Mobile communication device, system, and method | ⤷ Try a Trial |
| Mexico | 2014012811 | PREPARACION INYECTABLE. (INJECTABLE PREPARATION.) | ⤷ Try a Trial |
| Iceland | 7701 | Arípíprazól samsett lyfjaform og aðferð | ⤷ Try a Trial |
| China | 104376659 | MULTI-MODE COMMUNICATION INGESTIBLE EVENT MARKERS AND SYSTEMS, AND METHODS OF USING THE SAME | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for aripiprazole
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0367141 | SPC/GB04/039 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRAZOLE OR A SALT THEREOF; REGISTERED: UK EU/1/04/276/001 20040604; UK EU/1/04/276/002 20040604; UK EU/1/04/276/003 20040604; UK EU/1/04/276/004 20040604; UK EU/1/04/276/005 20040604; UK EU/1/04/276/006 20040604; UK EU/1/04/276/007 20040604; UK EU/1/04/276/008 20040604; UK EU/1/04/276/009 20040604; UK EU/1/04/276/010 20040604; UK EU/1/04/276/011 20040604; UK EU/1/04/276/012 20040604; UK EU/1/04/276/013 20040604; UK EU/1/04/276/014 20040604; UK EU/1/04/276/015 20040604; UK EU/1/04/276/016 20040604; UK EU/1/04/276/017 20040604; UK EU/1/04/276/018 20040604; UK EU/1/04/276/019 20040604; UK EU/1/04/276/020 20040604 |
| 1675573 | PA2014020 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRAZOLUM; REGISTRATION NO/DATE: EU/1/13/882 20131115 |
| 1675573 | 122014000057 | Germany | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRAZOL; REGISTRATION NO/DATE: EU/1/13/882 20131115 |
| 1675573 | C01675573/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRAZOL; REGISTRATION NO/DATE: SWISSMEDIC 63177 28.04.2014 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.