Paricalcitol - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for paricalcitol and what is the scope of freedom to operate?
Paricalcitol
is the generic ingredient in two branded drugs marketed by Amneal Pharms, Aurobindo Pharma Ltd, Bionpharma, Dr Reddys, Lotus Pharm Co Ltd, Marksans Pharma, Rising, Teva Pharms Usa, Abbvie, Accord Hlthcare, Amneal Pharms Co, Epic Pharma Llc, Eugia Pharma, Hikma Pharms, Hospira, and Sandoz, and is included in nineteen NDAs. Additional information is available in the individual branded drug profile pages.There are ten drug master file entries for paricalcitol. Twelve suppliers are listed for this compound. There is one tentative approval for this compound.
Summary for paricalcitol
| US Patents: | 0 |
| Tradenames: | 2 |
| Applicants: | 16 |
| NDAs: | 19 |
| Drug Master File Entries: | 10 |
| Finished Product Suppliers / Packagers: | 12 |
| Raw Ingredient (Bulk) Api Vendors: | 67 |
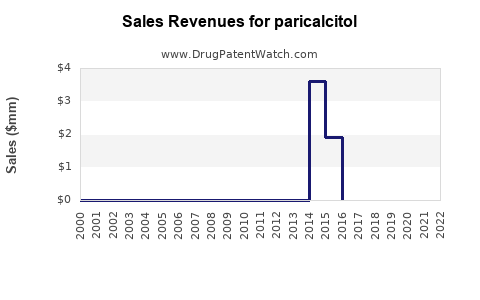
| Clinical Trials: | 96 |
| Patent Applications: | 2,865 |
| Formulation / Manufacturing: | see details |
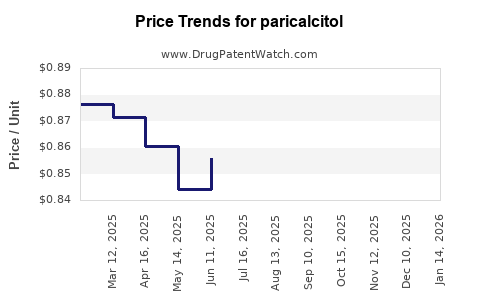
| Drug Prices: | Drug price trends for paricalcitol |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for paricalcitol |
| What excipients (inactive ingredients) are in paricalcitol? | paricalcitol excipients list |
| DailyMed Link: | paricalcitol at DailyMed |
Recent Clinical Trials for paricalcitol
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Cedars-Sinai Medical Center | Early Phase 1 |
| National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Early Phase 1 |
| Chengdu Suncadia Medicine Co., Ltd. | Phase 3 |
Generic filers with tentative approvals for PARICALCITOL
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 10MCG/ML | INJECTABLE; INJECTION |
| ⤷ Try a Trial | ⤷ Try a Trial | 5MCG/ML | INJECTABLE; INJECTION |
| ⤷ Try a Trial | ⤷ Try a Trial | 2MCG/ML | INJECTABLE; INJECTION |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Pharmacology for paricalcitol
| Drug Class | Vitamin D Analog Vitamin D2 Analog Vitamin D3 Analog |
Paragraph IV (Patent) Challenges for PARICALCITOL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ZEMPLAR | Injection | paricalcitol | 0.002 mg per mL in 1 mL vial and 0.005 mg per mL in 1 mL and 2 mL vials | 020819 | 1 | 2008-11-28 |
| ZEMPLAR | Capsules | paricalcitol | 1 mcg and 2 mcg | 021606 | 1 | 2008-10-14 |
| ZEMPLAR | Capsules | paricalcitol | 4 mcg | 021606 | 1 | 2008-08-25 |
US Patents and Regulatory Information for paricalcitol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rising | PARICALCITOL | paricalcitol | SOLUTION;INTRAVENOUS | 203897-003 | Nov 2, 2017 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Accord Hlthcare | PARICALCITOL | paricalcitol | SOLUTION;INTRAVENOUS | 207174-001 | Feb 4, 2016 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sandoz | PARICALCITOL | paricalcitol | SOLUTION;INTRAVENOUS | 091108-001 | Jul 27, 2011 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Eugia Pharma | PARICALCITOL | paricalcitol | SOLUTION;INTRAVENOUS | 205982-003 | Oct 9, 2018 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for paricalcitol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Abbvie | ZEMPLAR | paricalcitol | CAPSULE;ORAL | 021606-003 | May 26, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | ZEMPLAR | paricalcitol | CAPSULE;ORAL | 021606-003 | May 26, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | ZEMPLAR | paricalcitol | CAPSULE;ORAL | 021606-001 | May 26, 2005 | ⤷ Try a Trial | ⤷ Try a Trial |
| Abbvie | ZEMPLAR | paricalcitol | SOLUTION;INTRAVENOUS | 020819-001 | Apr 17, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.