Drug Price Trends for SOLIFENACIN
✉ Email this page to a colleague
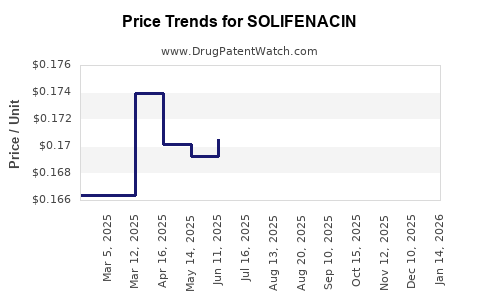
Average Pharmacy Cost for SOLIFENACIN
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| SOLIFENACIN 10 MG TABLET | 29300-0329-19 | 0.18102 | EACH | 2024-10-23 |
| SOLIFENACIN 10 MG TABLET | 31722-0028-30 | 0.18102 | EACH | 2024-10-23 |
| SOLIFENACIN 10 MG TABLET | 29300-0329-13 | 0.18102 | EACH | 2024-10-23 |
| SOLIFENACIN 10 MG TABLET | 31722-0028-90 | 0.18102 | EACH | 2024-10-23 |
| SOLIFENACIN 10 MG TABLET | 00591-3796-19 | 0.18102 | EACH | 2024-10-23 |
| SOLIFENACIN 5 MG TABLET | 72606-0009-01 | 0.21419 | EACH | 2024-10-23 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for SOLIFENACIN
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| SOLIFENACIN SUCCINATE 5MG TAB | AvKare, LLC | 69367-0295-09 | 90 | 31.26 | 0.34733 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| SOLIFENACIN SUCCINATE 5MG TAB | AvKare, LLC | 69367-0295-30 | 30 | 10.30 | 0.34333 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| SOLIFENACIN SUCCINATE 10MG TAB | AvKare, LLC | 69367-0296-09 | 90 | 33.21 | 0.36900 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| SOLIFENACIN SUCCINATE 10MG TAB | AvKare, LLC | 69367-0296-30 | 30 | 11.17 | 0.37233 | EACH | 2023-06-15 - 2028-06-14 | FSS |
| VESICARE 10MG TAB | Astellas Pharma U.S., Inc. | 51248-0151-01 | 30 | 249.85 | 8.32833 | EACH | 2023-01-01 - 2026-09-29 | FSS |
| VESICARE 5MG TAB | Astellas Pharma U.S., Inc. | 51248-0150-01 | 30 | 281.90 | 9.39667 | EACH | 2024-01-01 - 2026-09-29 | FSS |
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |


