Noven Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVEN, and what generic alternatives to NOVEN drugs are available?
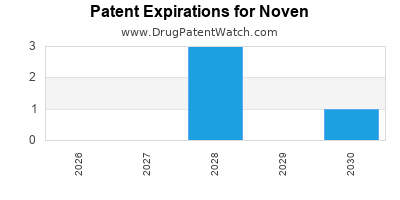
NOVEN has eight approved drugs.
There are eleven US patents protecting NOVEN drugs. There are three tentative approvals on NOVEN drugs.
There are fifty-four patent family members on NOVEN drugs in eighteen countries and fifty-two supplementary protection certificates in fourteen countries.
Summary for Noven
| International Patents: | 54 |
| US Patents: | 11 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 8 |
| Patent Litigation for Noven: | See patent lawsuits for Noven |
| PTAB Cases with Noven as petitioner: | See PTAB cases with Noven as petitioner |
Drugs and US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-004 | Mar 22, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-002 | Mar 22, 2022 | RX | Yes | No | 9,034,370 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-002 | Apr 6, 2006 | AB | RX | Yes | No | 9,668,981 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-002 | Mar 22, 2022 | RX | Yes | No | 11,559,501 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-001 | Mar 22, 2022 | RX | Yes | No | 11,559,501 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-002 | Mar 22, 2022 | RX | Yes | No | 9,456,993 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-001 | Mar 22, 2022 | RX | Yes | No | 8,591,941 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Noven | DENTIPATCH | lidocaine | PATCH;TOPICAL | 020575-002 | May 21, 1996 | 5,446,070 | ⤷ Try a Trial |
| Noven Pharms Inc | COMBIPATCH | estradiol; norethindrone acetate | FILM, EXTENDED RELEASE;TRANSDERMAL | 020870-002 | Aug 7, 1998 | 6,024,976 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-001 | Apr 6, 2006 | 5,958,446 | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-001 | Oct 29, 2012 | 6,024,976 | ⤷ Try a Trial |
| Noven | DENTIPATCH | lidocaine | FILM, EXTENDED RELEASE;BUCCAL | 020575-001 | May 21, 1996 | 5,446,070 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-002 | Apr 6, 2006 | 6,210,705 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-002 | Apr 6, 2006 | 6,348,211 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVEN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Transdermal System | 0.0375 mg/day, 0.05 mg/day, 0.075 mg/day, 0.1 mg/day | ➤ Subscribe | 2014-08-18 |
| ➤ Subscribe | Transdermal System | 10 mg/9 hrs, 15 mg/9 hrs, 20 mg/9 hrs and 30 mg/9 hrs | ➤ Subscribe | 2011-04-13 |
| ➤ Subscribe | Transdermal System | 0.025 mg/day | ➤ Subscribe | 2015-05-08 |
International Patents for Noven Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Taiwan | 201427723 | ⤷ Try a Trial |
| Argentina | 093118 | ⤷ Try a Trial |
| Taiwan | 201004630 | ⤷ Try a Trial |
| Argentina | 098878 | ⤷ Try a Trial |
| Mexico | 2007004315 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2006041911 | ⤷ Try a Trial |
| Peru | 20060491 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Noven Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2782584 | LUC00245 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210701 |
| 0402407 | 97C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL HEMIHYDRAAT; NAT. REGISTRATION NO/DATE: 298 IS 190 F 15 19960806; FIRST REGISTRATION: GB PL/0053/0241 19950711 |
| 2782584 | 132021000000197 | Italy | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLO (17SS-ESTRADIOLO) IN PARTICOLARE NELLA FORMA EMIIDRATA, E PROGESTERONE COMPRENDENTI LE VARIE FORME DI ESTRADIOLO (17SS-ESTRADIOLO), QUALI LE FORME IDRATE E SOLVATATE, INCLUDENDO LA FORMA EMIIDRATA, ED I SUOI SALI.(BIJUVA); AUTHORISATION NUMBER(S) AND DATE(S): BE582231, 20210406;048335018 -048335020, 20210517 |
| 1214076 | 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON; REGISTRATION NO/DATE: 1-27586 20080612 |
| 1453521 | C 2015 029 | Romania | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL SI ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: RO 7793/2015/001; DATE OF NATIONAL AUTHORISATION: 20150612; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SK. 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150129 |
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 0136011 | C00136011/03 | Switzerland | ⤷ Try a Trial | PRODUCT NMAE: ESTRADIOL UND MEDROXYPROGESTERONACETAT; REGISTRATION NO/DATE: IKS 55288 20000417 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.