Incyte Corp Company Profile
✉ Email this page to a colleague
What is the competitive landscape for INCYTE CORP, and what generic alternatives to INCYTE CORP drugs are available?
INCYTE CORP has three approved drugs.
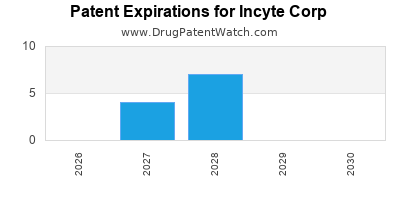
There are twenty-four US patents protecting INCYTE CORP drugs.
There are three hundred and ninety-six patent family members on INCYTE CORP drugs in fifty countries and forty-one supplementary protection certificates in eighteen countries.
Drugs and US Patents for Incyte Corp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-003 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-002 | Nov 16, 2011 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-004 | Nov 16, 2011 | RX | Yes | No | 8,722,693*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Incyte Corp | JAKAFI | ruxolitinib phosphate | TABLET;ORAL | 202192-001 | Nov 16, 2011 | RX | Yes | No | 9,079,912*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for INCYTE CORP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 5 mg, 10 mg, 15 mg, 20 mg, and 25 mg | ➤ Subscribe | 2015-12-17 |
International Patents for Incyte Corp Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Spain | 2543903 | ⤷ Try a Trial |
| Japan | 2023542137 | ⤷ Try a Trial |
| South Korea | 20100049010 | ⤷ Try a Trial |
| Denmark | 2455382 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Incyte Corp Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2861595 | 301131 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PEMIGATINIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/21/1535 20210329 |
| 2861595 | C20210023 00404 | Estonia | ⤷ Try a Trial | PRODUCT NAME: PEMIGATINIIB;REG NO/DATE: EU/1/21/1535 29.03.2021 |
| 2861595 | SPC/GB21/052 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: PEMIGATINIB OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF; REGISTERED: UK EU/1/21/1535(FOR NI) 20210329; UK FURTHER MA ON IPSUM 20210329 |
| 1966202 | C300574 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: RUXOLITINIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/12/773/001-003 20120823 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.