VIIV HLTHCARE Company Profile
✉ Email this page to a colleague
What is the competitive landscape for VIIV HLTHCARE, and what generic alternatives to VIIV HLTHCARE drugs are available?
VIIV HLTHCARE has twenty-six approved drugs.
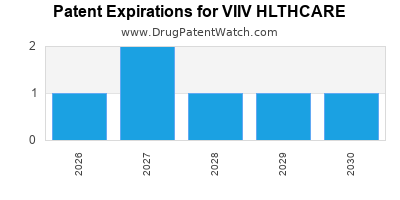
There are twelve US patents protecting VIIV HLTHCARE drugs.
There are five hundred and fourteen patent family members on VIIV HLTHCARE drugs in fifty-nine countries and two hundred and eleven supplementary protection certificates in eighteen countries.
Summary for VIIV HLTHCARE
| International Patents: | 514 |
| US Patents: | 12 |
| Tradenames: | 19 |
| Ingredients: | 17 |
| NDAs: | 26 |
Drugs and US Patents for VIIV HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | RUKOBIA | fostemsavir tromethamine | TABLET, EXTENDED RELEASE;ORAL | 212950-001 | Jul 2, 2020 | RX | Yes | Yes | 7,745,625 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Viiv Hlthcare | TIVICAY | dolutegravir sodium | TABLET;ORAL | 204790-003 | Jun 9, 2016 | DISCN | Yes | No | 9,242,986*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Viiv Hlthcare | VOCABRIA | cabotegravir sodium | TABLET;ORAL | 212887-001 | Jan 21, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Viiv Hlthcare | SELZENTRY | maraviroc | TABLET;ORAL | 022128-003 | Nov 4, 2016 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for VIIV HLTHCARE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Viiv Hlthcare | RETROVIR | zidovudine | TABLET;ORAL | 020518-002 | Oct 4, 1996 | 4,724,232 | ⤷ Try a Trial |
| Viiv Hlthcare | ZIAGEN | abacavir sulfate | SOLUTION;ORAL | 020978-001 | Dec 17, 1998 | 6,294,978*PED | ⤷ Try a Trial |
| Viiv Hlthcare | LEXIVA | fosamprenavir calcium | SUSPENSION;ORAL | 022116-001 | Jun 14, 2007 | 6,436,989*PED | ⤷ Try a Trial |
| Viiv Hlthcare | JULUCA | dolutegravir sodium; rilpivirine hydrochloride | TABLET;ORAL | 210192-001 | Nov 21, 2017 | 7,067,522 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for VIIV HLTHCARE drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 10 mg/mL | ➤ Subscribe | 2011-11-22 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-01-28 |
| ➤ Subscribe | Tablets | 700 mg | ➤ Subscribe | 2012-01-18 |
| ➤ Subscribe | Tablets | 600 mg/300 mg | ➤ Subscribe | 2007-09-27 |
| ➤ Subscribe | Tablets | 600 mg/50 mg/300 mg | ➤ Subscribe | 2017-08-14 |
| ➤ Subscribe | Tablets | 150 mg and 300 mg | ➤ Subscribe | 2007-10-16 |
| ➤ Subscribe | Tablets | 150 mg and 300 mg | ➤ Subscribe | 2011-08-08 |
| ➤ Subscribe | Oral Solution | 20 mg/ml | ➤ Subscribe | 2012-12-27 |
| ➤ Subscribe | Tablets | 150 mg/300 mg | ➤ Subscribe | 2007-06-26 |
| ➤ Subscribe | Tablets | 10 mg, 25 mg and 50 mg | ➤ Subscribe | 2017-08-14 |
| ➤ Subscribe | Tablets | 300 mg/150 mg/300 mg | ➤ Subscribe | 2011-03-22 |
International Patents for VIIV HLTHCARE Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 5317257 | ⤷ Try a Trial |
| Luxembourg | 92855 | ⤷ Try a Trial |
| Poland | 1874117 | ⤷ Try a Trial |
| Japan | 5247661 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for VIIV HLTHCARE Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1632232 | 300852 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN: - RILPIVIRINEHYDROCHLORIDE OF EEN THERAPEUTISCH EQUIVALENTE VORM DAARVAN ZOALS BESCHERMD DOOR HET BASISOCTROOI; - EMTRICITABINE; EN - TENOFOVIRALAFENAMIDE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, IN HET BIJZONDER TENOFOVIRALAFENAMIDEFUMARAAT; REGISTRATION NO/DATE: EU/1/16/1112 20160623 |
| 1284974 | PA2008004 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: MARAVIROCUM; REGISTRATION NO/DATE: EU/1/07/418/001 - EU/1/07/418/010 20070918 |
| 1419152 | 2012C/022 | Belgium | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRINE EN TENOFOVIR DISOPROXIL; AUTHORISATION NUMBER AND DATE: EU/1/11/737/001 20111128 |
| 1663240 | 92855 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE RILPIVIRINE OU UNE FORME THERAPEUTIQUE EQUIVALENTE QUI EN DERIVE TELLE QUE PROTEGEE PAR LE BREVET DE BASE, TEL QU'UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE RILPIVIRINE, INCLUANT LE SEL CHLORHYDRATE DE RILPIVIRINE , TENOFOVIR, EN PARTICULIER LE FUMARATE DE TENOFOVIR DISOPROXIL, ET L'EMTRICITABINE. |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.