Almirall Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ALMIRALL, and when can generic versions of ALMIRALL drugs launch?
ALMIRALL has twelve approved drugs.
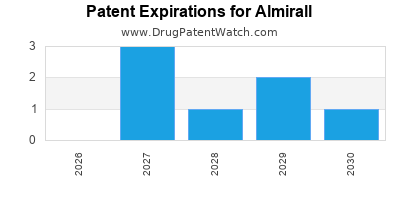
There are twenty US patents protecting ALMIRALL drugs.
There are two hundred and thirteen patent family members on ALMIRALL drugs in thirty-one countries and thirty-one supplementary protection certificates in fourteen countries.
Drugs and US Patents for Almirall
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almirall | CORDRAN | flurandrenolide | OINTMENT;TOPICAL | 012806-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Sign Up | ⤷ Sign Up | |||||
| Almirall | SEYSARA | sarecycline hydrochloride | TABLET;ORAL | 209521-003 | Oct 1, 2018 | RX | Yes | Yes | 9,255,068 | ⤷ Sign Up | Y | Y | ⤷ Sign Up | ||
| Almirall | AZELEX | azelaic acid | CREAM;TOPICAL | 020428-001 | Sep 13, 1995 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Almirall | KLISYRI | tirbanibulin | OINTMENT;TOPICAL | 213189-001 | Dec 14, 2020 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | |||||
| Almirall | ALTABAX | retapamulin | OINTMENT;TOPICAL | 022055-001 | Apr 12, 2007 | RX | Yes | Yes | 7,875,630 | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Almirall
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Almirall | XOLEGEL | ketoconazole | GEL;TOPICAL | 021946-001 | Jul 28, 2006 | 7,179,475 | ⤷ Sign Up |
| Almirall | CORDRAN | flurandrenolide | TAPE;TOPICAL | 016455-001 | Approved Prior to Jan 1, 1982 | 3,632,740 | ⤷ Sign Up |
| Almirall | VERDESO | desonide | AEROSOL, FOAM;TOPICAL | 021978-001 | Sep 19, 2006 | 6,730,288 | ⤷ Sign Up |
| Almirall | ALTABAX | retapamulin | OINTMENT;TOPICAL | 022055-001 | Apr 12, 2007 | RE43390 | ⤷ Sign Up |
| Almirall | XOLEGEL | ketoconazole | GEL;TOPICAL | 021946-001 | Jul 28, 2006 | 8,232,276 | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ALMIRALL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Gel | 7.5% | ➤ Subscribe | 2017-02-13 |
International Patents for Almirall Drugs
Supplementary Protection Certificates for Almirall Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435024 | 2021C/518 | Belgium | ⤷ Sign Up | PRODUCT NAME: UNE COMBINAISON DE FORMOTEROL (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), GLYCOPYRROLATE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET BUDESONIDE (Y COMPRIS TOUS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1498 20201210 |
| 1304992 | CR 2013 00053 | Denmark | ⤷ Sign Up | PRODUCT NAME: CLINDAMYCIN (SOM CLINDAMYCIN PHOSPHATE) OG TRETINOIN; NAT. REG. NO/DATE: 48954 20130416; FIRST REG. NO/DATE: IE PA1332/043/001 20130322 |
| 1836169 | C01836169/01 | Switzerland | ⤷ Sign Up | FORMER OWNER: ATHENEX, INC., US |
| 2435024 | LUC00208 | Luxembourg | ⤷ Sign Up | PRODUCT NAME: COMBINAISON DE FORMOTEROL (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES), DE GLYCOPYRRONIUM (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES) ET DE BUDESONIDE (Y COMPRIS SES SELS, ESTERS, SOLVATES OU ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES); AUTHORISATION NUMBER AND DATE: EU/1/20/1468 20201210 |
| 1304992 | 122013000081 | Germany | ⤷ Sign Up | PRODUCT NAME: CLINDAMYCIN (ALS CLINDAMYCIN-PHOSPHAT) UND TRETINOIN; NAT. REGISTRATION NO/DATE: 85210.00.00 20130611; FIRST REGISTRATION: IRLAND PA1332/043/001 20130322 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.