ASTELLAS Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ASTELLAS, and what generic alternatives to ASTELLAS drugs are available?
ASTELLAS has thirty-seven approved drugs.
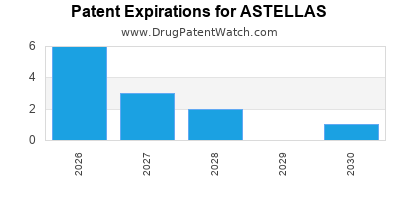
There are twenty-five US patents protecting ASTELLAS drugs. There is one tentative approval on ASTELLAS drugs.
There are four hundred and thirty-three patent family members on ASTELLAS drugs in forty-two countries and sixty-five supplementary protection certificates in nineteen countries.
Summary for ASTELLAS
| International Patents: | 433 |
| US Patents: | 25 |
| Tradenames: | 21 |
| Ingredients: | 16 |
| NDAs: | 37 |
| Patent Litigation for ASTELLAS: | See patent lawsuits for ASTELLAS |
Drugs and US Patents for ASTELLAS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astellas | MYCAMINE | micafungin sodium | INJECTABLE;INTRAVENOUS | 021506-003 | Jun 27, 2006 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Astellas | IZERVAY | avacincaptad pegol sodium | SOLUTION;INTRAVITREAL | 217225-001 | Aug 4, 2023 | RX | Yes | Yes | 9,617,546 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astellas | ARISTOCORT A | triamcinolone acetonide | CREAM;TOPICAL | 083015-003 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | ARISTOCORT | triamcinolone | TABLET;ORAL | 011161-010 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Astellas | CYCLOCORT | amcinonide | OINTMENT;TOPICAL | 018498-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ASTELLAS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astellas | VESICARE | solifenacin succinate | TABLET;ORAL | 021518-001 | Nov 19, 2004 | 6,017,927*PED | ⤷ Try a Trial |
| Astellas | VESICARE | solifenacin succinate | TABLET;ORAL | 021518-002 | Nov 19, 2004 | 6,017,927*PED | ⤷ Try a Trial |
| Astellas | ASTAGRAF XL | tacrolimus | CAPSULE, EXTENDED RELEASE;ORAL | 204096-002 | Jul 19, 2013 | 6,884,433 | ⤷ Try a Trial |
| Astellas | ADENOSCAN | adenosine | SOLUTION;INTRAVENOUS | 020059-001 | May 18, 1995 | 5,731,296 | ⤷ Try a Trial |
| Astellas | MYCAMINE | micafungin sodium | INJECTABLE;INTRAVENOUS | 021506-002 | Mar 16, 2005 | 6,265,536 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for ASTELLAS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Capsules | 0.5 mg, 1 mg, and 5 mg | ➤ Subscribe | 2013-11-15 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2014-06-16 |
| ➤ Subscribe | Injection | 3 mg/mL, 20 mL and 30 mL vials | ➤ Subscribe | 2005-04-16 |
| ➤ Subscribe | Tablets | 5 mg and 10 mg | ➤ Subscribe | 2009-04-08 |
| ➤ Subscribe | Capsules | 40 mg | ➤ Subscribe | 2016-08-31 |
| ➤ Subscribe | Injection | 3 mg/mL, 20 mL and 30 mL vials | ➤ Subscribe | 2005-04-18 |
| ➤ Subscribe | Injection | 0.08 mg/mL, 5 mL vial | ➤ Subscribe | 2012-04-10 |
International Patents for ASTELLAS Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2016515565 | ⤷ Try a Trial |
| Russian Federation | 2638833 | ⤷ Try a Trial |
| Russian Federation | 2448096 | ⤷ Try a Trial |
| Serbia | 52274 | ⤷ Try a Trial |
| Denmark | 2444085 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for ASTELLAS Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1280795 | 1690011-0 | Sweden | ⤷ Try a Trial | PRODUCT NAME: ISAVUCONAZOLE; REG. NO/DATE: EU/1/15/1036 20151016 |
| 2428508 | C02428508/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: GILTERITINIBUM; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67211 24.09.2020 |
| 2428508 | 2020/004 | Ireland | ⤷ Try a Trial | PRODUCT NAME: GILTERITINIB OR A SALT THEREOF; REGISTRATION NO/DATE: EU/1/19/1399 20191024 |
| 1893196 | 13C0071 | France | ⤷ Try a Trial | PRODUCT NAME: ENZALUTAMIDE OU SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/13/846 20130625 |
| 1280795 | 5/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: ISAVUCONAZOL ALS ISAVUCONAZONIUMSULFAT ODER ALS EIN ISAVUCONAZONIUMSALZ MIT EINEM ANDEREN PHARMAZEUTISCH ANNEHMBAREN ANION, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, HYDRATS ODER SOLVATS; REGISTRATION NO/DATE: EU/1/15/1036 (MITTEILUNG) 20151015 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.