Wyeth Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for WYETH PHARMS, and what generic alternatives to WYETH PHARMS drugs are available?
WYETH PHARMS has thirty-one approved drugs.
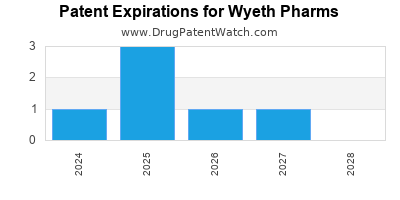
There are six US patents protecting WYETH PHARMS drugs.
There are fifty-six patent family members on WYETH PHARMS drugs in thirty countries and thirty-six supplementary protection certificates in thirteen countries.
Drugs and US Patents for Wyeth Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wyeth Pharms | PREMARIN | estrogens, conjugated | TABLET;ORAL | 004782-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms | PREMARIN | estrogens, conjugated | TABLET;ORAL | 004782-004 | Approved Prior to Jan 1, 1982 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms Inc | PIPRACIL | piperacillin sodium | INJECTABLE;INJECTION | 050545-003 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms Inc | LODINE | etodolac | TABLET;ORAL | 018922-004 | Jul 29, 1993 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms | PROTONIX | pantoprazole sodium | FOR SUSPENSION, DELAYED RELEASE;ORAL | 022020-001 | Nov 14, 2007 | AB | RX | Yes | Yes | 7,550,153*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Wyeth Pharms Inc | ORUVAIL | ketoprofen | CAPSULE, EXTENDED RELEASE;ORAL | 019816-003 | Feb 8, 1995 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Wyeth Pharms | CORDARONE | amiodarone hydrochloride | TABLET;ORAL | 018972-001 | Dec 24, 1985 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Wyeth Pharms
Paragraph IV (Patent) Challenges for WYETH PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | For Injection | 12 g/1.5 g per vial (pharmacy bulk) | ➤ Subscribe | 2011-12-06 |
| ➤ Subscribe | Tablets | 0.09 mg/0.02 mg | ➤ Subscribe | 2007-10-05 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg and 40 mg | ➤ Subscribe | 2004-02-02 |
| ➤ Subscribe | Tablets | 25 mg, 37.5 mg, 50 mg, 75 mg and 100 mg | ➤ Subscribe | 2005-11-03 |
International Patents for Wyeth Pharms Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Poland | 1667660 | ⤷ Try a Trial |
| Norway | 339714 | ⤷ Try a Trial |
| South Africa | 200602682 | ⤷ Try a Trial |
| Japan | 4789806 | ⤷ Try a Trial |
| Taiwan | 200524643 | ⤷ Try a Trial |
| Panama | 8629101 | ⤷ Try a Trial |
| China | 1938272 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Wyeth Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 1453521 | 39/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 0166287 | 96C0032 | Belgium | ⤷ Try a Trial | PRODUCT NAME: PANTOPRAZOL. NATR. SESQUIHYDRAS PANTOPRAZOLE; NAT. REGISTRATION NO/DATE: 127 IS 98 F 3 19960222; FIRST REGISTRATION: SE 12131 19940506 |
| 0802183 | 2009/028 | Ireland | ⤷ Try a Trial | PRODUCT NAME: BAZEDOXIFENE AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/09/511/001-004 20090417 |
| 0771217 | 07C0001 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 1453521 | C 2015 029 | Romania | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL SI ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: RO 7793/2015/001; DATE OF NATIONAL AUTHORISATION: 20150612; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SK. 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150129 |
| 0136011 | 2000C/027 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOLUM / NORETHISTERONI ACETAS; NAT. REGISTRATION NO/DATE: 19 IS 106 F3 20000911; FIRST REGISTRATION: NL RVG 23909 19991124 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.