Sanofi Aventis Us Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANOFI AVENTIS US, and when can generic versions of SANOFI AVENTIS US drugs launch?
SANOFI AVENTIS US has one hundred and twenty-two approved drugs.
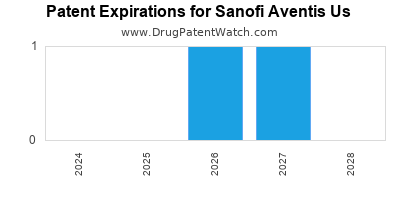
There are ten US patents protecting SANOFI AVENTIS US drugs.
There are two hundred and seventeen patent family members on SANOFI AVENTIS US drugs in fifty-two countries and one hundred and fifteen supplementary protection certificates in nineteen countries.
Summary for Sanofi Aventis Us
| International Patents: | 217 |
| US Patents: | 10 |
| Tradenames: | 97 |
| Ingredients: | 85 |
| NDAs: | 122 |
Drugs and US Patents for Sanofi Aventis Us
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | ARALEN PHOSPHATE W/ PRIMAQUINE PHOSPHATE | chloroquine phosphate; primaquine phosphate | TABLET;ORAL | 014860-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | MULTAQ | dronedarone hydrochloride | TABLET;ORAL | 022425-001 | Jul 1, 2009 | AB | RX | Yes | Yes | 9,107,900 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sanofi Aventis Us | HYDROXYSTILBAMIDINE ISETHIONATE | hydroxystilbamidine isethionate | INJECTABLE;INJECTION | 009166-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | DV | dienestrol | CREAM;VAGINAL | 083518-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | AUBAGIO | teriflunomide | TABLET;ORAL | 202992-001 | Sep 12, 2012 | AB | RX | Yes | No | 8,802,735*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Sanofi Aventis Us | DORIDEN | glutethimide | TABLET;ORAL | 009519-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Sanofi Aventis Us
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | BRICANYL | terbutaline sulfate | TABLET;ORAL | 017618-002 | Approved Prior to Jan 1, 1982 | 3,937,838 | ⤷ Try a Trial |
| Sanofi Aventis Us | TALWIN NX | naloxone hydrochloride; pentazocine hydrochloride | TABLET;ORAL | 018733-001 | Dec 16, 1982 | 4,105,659 | ⤷ Try a Trial |
| Sanofi Aventis Us | PLAVIX | clopidogrel bisulfate | TABLET;ORAL | 020839-001 | Nov 17, 1997 | 4,847,265*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | TAXOTERE | docetaxel | INJECTABLE;INJECTION | 020449-003 | Aug 3, 2010 | 4,814,470*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | LOVENOX | enoxaparin sodium | INJECTABLE;INTRAVENOUS, SUBCUTANEOUS | 020164-009 | Jan 23, 2003 | RE38743 | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | 5,698,582 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANOFI AVENTIS US drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 6.25 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Tablets | 7 mg and 14 mg | ➤ Subscribe | 2016-09-12 |
| ➤ Subscribe | Tablets | 300 mg/25 mg | ➤ Subscribe | 2006-06-06 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2013-07-01 |
| ➤ Subscribe | Injection | 5 mg/mL, 10 mL and 20 mL vials | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 200 mg/40 mL | ➤ Subscribe | 2007-07-16 |
| ➤ Subscribe | Injection | 100 mg/mL, 3 mL vials | ➤ Subscribe | 2006-12-07 |
| ➤ Subscribe | Extended-release Tablets | 12.5 mg | ➤ Subscribe | 2006-01-19 |
| ➤ Subscribe | Tablets | 150 mg/12.5 mg and 300 mg/12.5 mg | ➤ Subscribe | 2004-11-10 |
| ➤ Subscribe | Tablets | 75 mg, 150 mg and 300 mg | ➤ Subscribe | 2004-05-25 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-03-04 |
| ➤ Subscribe | Injection | 40 mg/mL, 0.5 mL and 2 mL vials | ➤ Subscribe | 2009-06-30 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 5 mg/mL, 40 mL vial | ➤ Subscribe | 2011-03-23 |
International Patents for Sanofi Aventis Us Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2443285 | ⤷ Try a Trial |
| New Zealand | 545835 | ⤷ Try a Trial |
| Tunisia | 2012000188 | ⤷ Try a Trial |
| Japan | 6182510 | ⤷ Try a Trial |
| Denmark | 1381356 | ⤷ Try a Trial |
| Japan | 2013505213 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Sanofi Aventis Us Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2768484 | SPC/GB19/067 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF DAUNORUBICIN AND CYTARABINE; REGISTERED: UK EU/1/18/1308(FOR NI) 20180827; UK PLGB 31626/0004 20180827 |
| 0454511 | C980039 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: IRBESARTAN, DESGEWENST IN DE VORM VAN EEN ZOUT EN/OF EEN HYDRAA T; REGISTRATION NO/DATE: EU/1/97/046/001 - 009 19970827 |
| 1381356 | 14C0010 | France | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMIDE,SON STEREOISOMERE ET LEURS SELS PHARMACEUTIQUEMENT ACCEPTABLES.; REGISTRATION NO/DATE: EU/1/13/838/001-005 20130826 |
| 3300601 | 2290030-2 | Sweden | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF DAUNORUBICIN AND CYTARABINE; REG. NO/DATE: EU/1/18/1308 20180827 |
| 1667986 | 636 | Finland | ⤷ Try a Trial | |
| 1667986 | 1390025-3 | Sweden | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXEL ACETONSOLVAT; REG. NO/DATE: EU/1/11/676/001 20110317 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.