Haleon Us Holdings Company Profile
✉ Email this page to a colleague
What is the competitive landscape for HALEON US HOLDINGS, and when can generic versions of HALEON US HOLDINGS drugs launch?
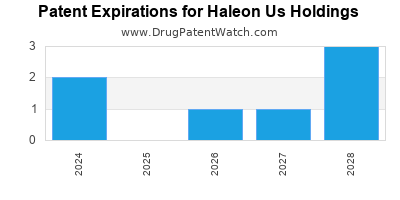
HALEON US HOLDINGS has thirty-one approved drugs.
There are ten US patents protecting HALEON US HOLDINGS drugs.
There are two hundred and three patent family members on HALEON US HOLDINGS drugs in thirty-two countries and sixteen supplementary protection certificates in eight countries.
Summary for Haleon Us Holdings
| International Patents: | 203 |
| US Patents: | 10 |
| Tradenames: | 28 |
| Ingredients: | 18 |
| NDAs: | 31 |
Drugs and US Patents for Haleon Us Holdings
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Haleon Us Holdings | NICORETTE | nicotine polacrilex | TROCHE/LOZENGE;ORAL | 022360-002 | May 18, 2009 | OTC | Yes | Yes | 8,501,164 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Haleon Us Holdings | ADVIL ALLERGY AND CONGESTION RELIEF | chlorpheniramine maleate; ibuprofen; phenylephrine hydrochloride | TABLET;ORAL | 022113-001 | Dec 21, 2011 | OTC | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Haleon Us Holdings | NICORETTE | nicotine polacrilex | TROCHE/LOZENGE;ORAL | 022360-001 | May 18, 2009 | OTC | Yes | No | 8,940,772 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Haleon Us Holdings
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Haleon Us Holdings | ADVIL CONGESTION RELIEF | ibuprofen; phenylephrine hydrochloride | TABLET;ORAL | 022565-001 | May 27, 2010 | 5,087,454 | ⤷ Try a Trial |
| Haleon Us Holdings | ABREVA | docosanol | CREAM;TOPICAL | 020941-001 | Jul 25, 2000 | 4,874,794 | ⤷ Try a Trial |
| Haleon Us Holdings | ADVIL PM | diphenhydramine hydrochloride; ibuprofen | CAPSULE;ORAL | 021393-001 | Dec 21, 2005 | 8,883,849 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for HALEON US HOLDINGS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Capsules | 200 mg/38 mg | ➤ Subscribe | 2016-02-16 |
| ➤ Subscribe | Gum | 2 mg | ➤ Subscribe | 2013-01-22 |
| ➤ Subscribe | Capsules | 200 mg/30 mg | ➤ Subscribe | 2004-12-27 |
| ➤ Subscribe | Capsules | 60 mg | ➤ Subscribe | 2010-09-08 |
| ➤ Subscribe | Gum | 4 mg | ➤ Subscribe | 2013-01-22 |
International Patents for Haleon Us Holdings Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Denmark | 1888042 | ⤷ Try a Trial |
| Australia | 2011200912 | ⤷ Try a Trial |
| Canada | 2559099 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Haleon Us Holdings Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1305329 | 08C0014 | France | ⤷ Try a Trial | PRODUCT NAME: FLUTICASONE FUROATE; REGISTRATION NO/DATE: EU/1/07/434/001 20080111 |
| 0428642 | 57/2007 | Austria | ⤷ Try a Trial | PRODUCT NAME: DOCOSANOL; NAT. REGISTRATION NO/DATE: 1-27014 20070518; FIRST REGISTRATION: SE 18517 20031114 |
| 0129748 | 98C0042 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ORLISTAT; REGISTRATION NO/DATE: EU/1/98/071/001 19980729 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.