Bionpharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BIONPHARMA, and when can generic versions of BIONPHARMA drugs launch?
BIONPHARMA has sixty-six approved drugs.
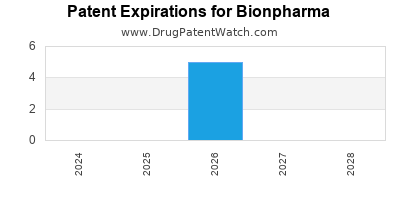
There are five US patents protecting BIONPHARMA drugs.
There are ten patent family members on BIONPHARMA drugs in thirteen countries and one hundred and seven supplementary protection certificates in fifteen countries.
Summary for Bionpharma
| International Patents: | 10 |
| US Patents: | 5 |
| Tradenames: | 61 |
| Ingredients: | 56 |
| NDAs: | 66 |
| Patent Litigation for Bionpharma: | See patent lawsuits for Bionpharma |
Drugs and US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bionpharma | PROCHLORPERAZINE MALEATE | prochlorperazine maleate | TABLET;ORAL | 217478-001 | Apr 4, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | PARICALCITOL | paricalcitol | CAPSULE;ORAL | 202539-003 | Mar 27, 2014 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | LORATADINE | loratadine | CAPSULE;ORAL | 202538-001 | Dec 21, 2018 | OTC | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bionpharma | FLUVOXAMINE MALEATE | fluvoxamine maleate | TABLET;ORAL | 217917-002 | Jan 22, 2024 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | NIMODIPINE | nimodipine | CAPSULE;ORAL | 076740-001 | Jan 17, 2008 | AB | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bionpharma | STAVZOR | valproic acid | CAPSULE, DELAYED RELEASE;ORAL | 022152-003 | Jul 29, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bionpharma | ENALAPRIL MALEATE | enalapril maleate | SOLUTION;ORAL | 212408-001 | Aug 10, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bionpharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bionpharma | MIDOL LIQUID GELS | ibuprofen | CAPSULE;ORAL | 021472-001 | Oct 18, 2002 | 6,251,426 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Bionpharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 101166520 | ⤷ Try a Trial |
| Cyprus | 1118321 | ⤷ Try a Trial |
| Denmark | 1863458 | ⤷ Try a Trial |
| European Patent Office | 1863458 | ⤷ Try a Trial |
| Portugal | 1863458 | ⤷ Try a Trial |
| European Patent Office | 3061447 | ⤷ Try a Trial |
| Mexico | 2007011039 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bionpharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1427415 | C300500 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: APIXABAN DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/11/691/001-005 20110518 |
| 0716606 | C300428 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER CARBONAAT OF BICARBONAAT; REGISTRATION NO/DATE: EU/1/09/521/001-007 20090610 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 0627406 | 11C0021 | France | ⤷ Try a Trial | PRODUCT NAME: FINGOLIMOD ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES EN PARTICULIER SON CHLORHYDRATE; REGISTRATION NO/DATE: EU/1/11/677/001 20110317 |
| 0162036 | C300028 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVETIRACETAM; REGISTRATION NO/DATE: EU/1/00/146/001 - EU/1/00/146/026 20000929 |
| 0480717 | 98C0025 | Belgium | ⤷ Try a Trial | PRODUCT NAME: LOSARTAN POTASSIUM; HYDROCHLOROTHIAZIDE; NAT. REGISTRATION NO/DATE: NL 20 037 19950215; FIRST REGISTRATION: FR - NL 20 037 19950215 |
| 1411900 | C300481 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: NAPROXEN EN ESOMEPRAZOL; NAT. REGISTRATION NO/DATE: RVG 106235 20101118; FIRST REGISTRATION: PL 17901/0263-001 20101105 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.