RAYALDEE Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Rayaldee, and what generic alternatives are available?
Rayaldee is a drug marketed by Eirgen and is included in one NDA. There are sixteen patents protecting this drug.
This drug has one hundred and seventy-two patent family members in thirty-six countries.
The generic ingredient in RAYALDEE is calcifediol. There are two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the calcifediol profile page.
DrugPatentWatch® Generic Entry Outlook for Rayaldee
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be March 14, 2034. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for RAYALDEE
| International Patents: | 172 |
| US Patents: | 16 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 76 |
| Clinical Trials: | 1 |
| Patent Applications: | 3,420 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for RAYALDEE |
| What excipients (inactive ingredients) are in RAYALDEE? | RAYALDEE excipients list |
| DailyMed Link: | RAYALDEE at DailyMed |

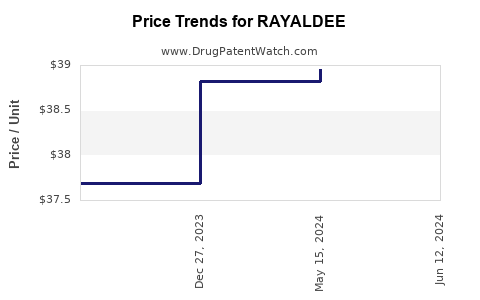
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for RAYALDEE
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for RAYALDEE
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| OPKO Health, Inc. | Phase 2 |
Pharmacology for RAYALDEE
| Drug Class | Vitamin D3 Analog |
Anatomical Therapeutic Chemical (ATC) Classes for RAYALDEE
US Patents and Regulatory Information for RAYALDEE
RAYALDEE is protected by twenty-two US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of RAYALDEE is ⤷ Sign Up.
This potential generic entry date is based on patent ⤷ Sign Up.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting RAYALDEE
Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D.sub.2 and 25-hydroxyvitamin D.sub.3
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Stabilized modified release vitamin D formulation and method of administering same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Stabilized modified release vitamin D formulation and method of administering same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Methods for controlled release oral dosage of a vitamin D compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SECONDARY HYPERPARATHYROIDISM IN STAGE 3 OR 4 CKD WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D TO REDUCE THE PATIENT'S SERUM PARATHYROID HORMONE LEVEL WHILE AVOIDING PTH OVERSUPPRESSION
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SECONDARY HYPERPARATHYROIDISM WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D IN CHRONIC KIDNEY DISEASE PATIENTS RECEIVING CHOLESTYRAMINE
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SECONDARY HYPERPARATHYROIDISM WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D IN CHRONIC KIDNEY DISEASE PATIENTS RECEIVING PHENOBARBITAL OR OTHER ANTICONVULSANTS
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SECONDARY HYPERPARATHYROIDISM WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D IN CHRONIC KIDNEY DISEASE PATIENTS RECEIVING CYP3A INHIBITORS
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATMENT OF SECONDARY HYPERPARATHYROIDISM IN PATIENTS WITH STAGE 3 OR 4 CHRONIC KIDNEY DISEASE USING CONTROLLED RELEASE, ORAL 25-HYDROXYVITAMIN D
Methods and compositions for controlled release oral dosage of a vitamin D compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D.sub.2 and 25-hydroxyvitamin D.sub.3
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF SUSTAINED RELEASE 25-HYDROXYVITAMIN D IN TREATING PATIENTS HAVING 25-HYDROXYVITAMIN D INSUFFICIENCY OR DEFICIENCY
Methods for controlled release oral dosage of a vitamin D compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: ADMINISTRATION OF 25-HYDROXYVITAMIN D3 BY CONTROLLED RELEASE
Oral dosage form of 25-hydroxyvitamin D
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF CONTROLLED RELEASE 25-HYDROXYVITAMIN D IN TREATING SECONDARY HYPERPARATHYROIDISM IN PATIENTS HAVING CHRONIC KIDNEY DISEASE
Method for controlled release oral dosage of a vitamin D compound
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: USE OF SUSTAINED OR EXTENDED RELEASE ORAL 25-HYDROXYVITAMIN D3 IN TREATING SECONDARY HYPERPARATHROIDISM IN ADULT PATIENTS HAVING CHRONIC KIDNEY DISEASE STAGE 3 OR 4
Stabilized modified release vitamin D formulation and method of administering same
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SECONDARY HYPERPARATHYROIDISM IN CKD WITH SUSTAINED RELEASE CALCIFEDIOL TO REDUCE THE PATIENT'S SERUM PARATHYROID HORMONE LEVEL AND CMAX24HR/C24HR IS REDUCED COMPARED TO BOLUS IV INJECTION AND IMMEDIATE-RELEASE, ORAL DOSING
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SECONDARY HYPERPARATHYROIDISM IN CHRONIC KIDNEY DISEASE WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D TO REDUCE THE PATIENT'S SERUM PARATHYROID HORMONE LEVEL AND THE SUSTAINED RELEASE IS OVER AT LEAST 10 HOURS
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SECONDARY HYPERPARATHYROIDISM IN CHRONIC KIDNEY DISEASE WITH SUSTAINED RELEASE 25-HYDROXYVITAMIN D TO REDUCE THE PATIENT'S SERUM PARATHYROID HORMONE LEVEL AND CMAX IS REDUCED COMPARED TO BOLUS IV INJECTION AND IMMEDIATE-RELEASE, ORAL DOSING
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SHPT IN CKD WITH SUSTAINED RELEASE CALCIFEDIOL TO REDUCE SERUM PARATHYROID HORMONE LEVEL AND CHANGE IN SERUM CONCENTRATION OF CALCIFEDIOL IN DOSE INTERVAL IS REDUCED COMPARED TO BOLUS IV INJECTION AND IMMEDIATE-RELEASE, ORAL DOSING
Method for treating secondary hyperparathyroidism in CKD
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: TREATING SECONDARY HYPERPARATHYROIDISM IN CKD WITH SUSTAINED RELEASE CALCIFEDIOL TO REDUCE THE PATIENT'S SERUM PARATHYROID HORMONE LEVEL AND TMAX IS INCREASED COMPARED TO BOLUS IV INJECTION AND IMMEDIATE-RELEASE, ORAL DOSING
Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3
Patent Number: ⤷ Sign Up
Patent Expiration: ⤷ Sign Up
Patented Use: MAINTAINING SERUM 25-HYDROXYVITAMIN D AT A LEVEL OF AT LEAST 30 NG/ML WITH ORAL, SUSTAINED RELEASE 25-HYDROXYVITAMIN D
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | Y | ⤷ Sign Up | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RAYALDEE
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eirgen | RAYALDEE | calcifediol | CAPSULE, EXTENDED RELEASE;ORAL | 208010-001 | Jun 17, 2016 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for RAYALDEE
When does loss-of-exclusivity occur for RAYALDEE?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5576
Estimated Expiration: ⤷ Sign Up
Australia
Patent: 14228069
Estimated Expiration: ⤷ Sign Up
Patent: 19200268
Estimated Expiration: ⤷ Sign Up
Brazil
Patent: 2015023658
Estimated Expiration: ⤷ Sign Up
Canada
Patent: 05409
Estimated Expiration: ⤷ Sign Up
Chile
Patent: 15002659
Estimated Expiration: ⤷ Sign Up
China
Patent: 5246464
Estimated Expiration: ⤷ Sign Up
Patent: 1346071
Estimated Expiration: ⤷ Sign Up
Costa Rica
Patent: 190178
Estimated Expiration: ⤷ Sign Up
Croatia
Patent: 0201284
Estimated Expiration: ⤷ Sign Up
Patent: 0201869
Estimated Expiration: ⤷ Sign Up
Patent: 0211265
Estimated Expiration: ⤷ Sign Up
Cyprus
Patent: 23167
Estimated Expiration: ⤷ Sign Up
Patent: 23568
Estimated Expiration: ⤷ Sign Up
Patent: 24393
Estimated Expiration: ⤷ Sign Up
Denmark
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
Ecuador
Patent: 23024864
Estimated Expiration: ⤷ Sign Up
Eurasian Patent Organization
Patent: 8867
Estimated Expiration: ⤷ Sign Up
Patent: 1591809
Estimated Expiration: ⤷ Sign Up
Patent: 1991774
Estimated Expiration: ⤷ Sign Up
European Patent Office
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
Patent: 88638
Estimated Expiration: ⤷ Sign Up
Germany
Patent: 2014011525
Estimated Expiration: ⤷ Sign Up
Hong Kong
Patent: 20128
Estimated Expiration: ⤷ Sign Up
Patent: 20362
Estimated Expiration: ⤷ Sign Up
Patent: 56895
Estimated Expiration: ⤷ Sign Up
Hungary
Patent: 51923
Estimated Expiration: ⤷ Sign Up
Patent: 52014
Estimated Expiration: ⤷ Sign Up
Patent: 55591
Estimated Expiration: ⤷ Sign Up
Israel
Patent: 1456
Estimated Expiration: ⤷ Sign Up
Patent: 4841
Estimated Expiration: ⤷ Sign Up
Japan
Patent: 92051
Estimated Expiration: ⤷ Sign Up
Patent: 33268
Estimated Expiration: ⤷ Sign Up
Patent: 82832
Estimated Expiration: ⤷ Sign Up
Patent: 16517429
Estimated Expiration: ⤷ Sign Up
Patent: 18012737
Estimated Expiration: ⤷ Sign Up
Patent: 19135264
Estimated Expiration: ⤷ Sign Up
Patent: 21155460
Estimated Expiration: ⤷ Sign Up
Lithuania
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
Malaysia
Patent: 4092
Estimated Expiration: ⤷ Sign Up
Mexico
Patent: 15012625
Estimated Expiration: ⤷ Sign Up
Patent: 20011736
Estimated Expiration: ⤷ Sign Up
New Zealand
Patent: 1924
Estimated Expiration: ⤷ Sign Up
Norway
Patent: 21007
Estimated Expiration: ⤷ Sign Up
Peru
Patent: 151761
Estimated Expiration: ⤷ Sign Up
Philippines
Patent: 015502162
Estimated Expiration: ⤷ Sign Up
Patent: 021551127
Estimated Expiration: ⤷ Sign Up
Poland
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
Portugal
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
Saudi Arabia
Patent: 5361134
Estimated Expiration: ⤷ Sign Up
Serbia
Patent: 846
Estimated Expiration: ⤷ Sign Up
Patent: 132
Estimated Expiration: ⤷ Sign Up
Patent: 176
Estimated Expiration: ⤷ Sign Up
Singapore
Patent: 201703517V
Estimated Expiration: ⤷ Sign Up
Patent: 201507323P
Estimated Expiration: ⤷ Sign Up
Slovenia
Patent: 68172
Estimated Expiration: ⤷ Sign Up
Patent: 32773
Estimated Expiration: ⤷ Sign Up
Patent: 50016
Estimated Expiration: ⤷ Sign Up
South Korea
Patent: 1847947
Estimated Expiration: ⤷ Sign Up
Patent: 2203003
Estimated Expiration: ⤷ Sign Up
Patent: 140113374
Estimated Expiration: ⤷ Sign Up
Patent: 140140004
Estimated Expiration: ⤷ Sign Up
Patent: 190095216
Estimated Expiration: ⤷ Sign Up
Patent: 210078463
Estimated Expiration: ⤷ Sign Up
Spain
Patent: 09477
Estimated Expiration: ⤷ Sign Up
Patent: 34900
Estimated Expiration: ⤷ Sign Up
Patent: 82567
Estimated Expiration: ⤷ Sign Up
Taiwan
Patent: 59753
Estimated Expiration: ⤷ Sign Up
Patent: 1707689
Estimated Expiration: ⤷ Sign Up
Ukraine
Patent: 3386
Estimated Expiration: ⤷ Sign Up
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering RAYALDEE around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Portugal | 2148661 | ⤷ Sign Up | |
| European Patent Office | 3225243 | PROCÉDÉ SÛR ET EFFICACE DE TRAITEMENT ET DE PRÉVENTION DE L'HYPERPARATHYROÏDISME SECONDAIRE DANS UNE MALADIE RÉNALE CHRONIQUE (METHOD OF SAFELY AND EFFECTIVELY TREATING AND PREVENTING SECONDARY HYPERPARATHYROIDISM IN CHRONIC KIDNEY DISEASE) | ⤷ Sign Up |
| Japan | 2010525078 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RAYALDEE
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2481400 | 132021000000071 | Italy | ⤷ Sign Up | PRODUCT NAME: CALCIFEDIOLO(RAYALDEE); AUTHORISATION NUMBER(S) AND DATE(S): 047870011, 20201201;PL 50784/0005, 20200721 |
| 2481400 | 122020000079 | Germany | ⤷ Sign Up | PRODUCT NAME: CALCIFEDIOL UND/ODER PHARMAZEUTISCH AKZEPTABLE SALZE UND HYDRATE DAVON, BEVORZUGT CALCIFEDIOLMONOHYDRAT; NAT. REGISTRATION NO/DATE: 2202115.00.00 20200818; FIRST REGISTRATION: VEREINIGTES KOENIGREICH GROSSBRITANNIEN UND NORDIRLAND PL 50784/0005 - 0001 20200721 |
| 2481400 | C202130022 | Spain | ⤷ Sign Up | PRODUCT NAME: CALCIFEDIOL; NATIONAL AUTHORISATION NUMBER: 85519-DE/H/5590/001/DC; DATE OF AUTHORISATION: 20201230; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): DE/H/5590/001/DC; DATE OF FIRST AUTHORISATION IN EEA: 20200721 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


