Woodward Company Profile
✉ Email this page to a colleague
What is the competitive landscape for WOODWARD, and what generic alternatives to WOODWARD drugs are available?
WOODWARD has fourteen approved drugs.
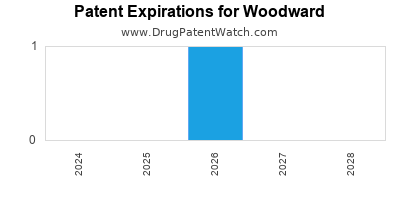
There is one US patent protecting WOODWARD drugs.
There are twenty-four patent family members on WOODWARD drugs in fourteen countries and fourteen supplementary protection certificates in five countries.
Drugs and US Patents for Woodward
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Woodward | COREG CR | carvedilol phosphate | CAPSULE, EXTENDED RELEASE;ORAL | 022012-003 | Oct 20, 2006 | AB | RX | Yes | Yes | 8,101,209*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Woodward | AVODART | dutasteride | CAPSULE;ORAL | 021319-001 | Nov 20, 2001 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Woodward | DAPIPRAZOLE HYDROCHLORIDE | dapiprazole hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 204902-001 | May 30, 2019 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-003 | Dec 15, 2004 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Woodward | AVANDIA | rosiglitazone maleate | TABLET;ORAL | 021071-003 | May 25, 1999 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Woodward Specl | DECA-DURABOLIN | nandrolone decanoate | INJECTABLE;INJECTION | 013132-002 | Jun 12, 1986 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Woodward
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Woodward | LUNESTA | eszopiclone | TABLET;ORAL | 021476-001 | Dec 15, 2004 | 7,381,724*PED | ⤷ Try a Trial |
| Woodward | AVANDIA | rosiglitazone maleate | TABLET;ORAL | 021071-004 | May 25, 1999 | 5,002,953*PED | ⤷ Try a Trial |
| Woodward | COREG | carvedilol | TABLET;ORAL | 020297-003 | Sep 14, 1995 | 4,503,067*PED | ⤷ Try a Trial |
| Woodward | AVANDIA | rosiglitazone maleate | TABLET;ORAL | 021071-002 | May 25, 1999 | 5,741,803*PED | ⤷ Try a Trial |
| Woodward | COREG | carvedilol | TABLET;ORAL | 020297-003 | Sep 14, 1995 | 5,760,069*PED | ⤷ Try a Trial |
| Woodward | COREG CR | carvedilol phosphate | CAPSULE, EXTENDED RELEASE;ORAL | 022012-004 | Oct 20, 2006 | RE40000*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for WOODWARD drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Capsules | 10 mg and 20 mg | ➤ Subscribe | 2008-03-18 |
| ➤ Subscribe | Extended-release Capsules | 80 mg | ➤ Subscribe | 2007-11-19 |
| ➤ Subscribe | Capsule | 0.5 mg/0.4 mg | ➤ Subscribe | 2010-10-26 |
| ➤ Subscribe | Tablets | 1 mg, 2 mg and 3 mg | ➤ Subscribe | 2008-12-15 |
| ➤ Subscribe | Extended-release Capsules | 40 mg | ➤ Subscribe | 2007-12-21 |
| ➤ Subscribe | Capsules | 0.5 mg | ➤ Subscribe | 2007-10-29 |
| ➤ Subscribe | Capsules | 1 g | ➤ Subscribe | 2008-11-10 |
International Patents for Woodward Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Brazil | PI0213175 | ⤷ Try a Trial |
| Canada | 2463134 | ⤷ Try a Trial |
| France | 2830447 | ⤷ Try a Trial |
| Japan | 4718116 | ⤷ Try a Trial |
| Mexico | PA04003367 | ⤷ Try a Trial |
| Brazil | 0213175 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Woodward Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0268956 | 1999C0030 | Belgium | ⤷ Try a Trial | PRODUCT NAME: RABEPRAZOLE SODIUM; NAT. REGISTRATION NO/DATE: 5532 IE 1 F 3 19990201; FIRST REGISTRATION: GB 10555/0010 19980508 |
| 0658161 | C300035 | Netherlands | ⤷ Try a Trial | PRODUCT: ROSIGLITAZONE,MALEAAT, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR SOLVAAT. |
| 0268956 | SPC/GB98/040 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: RABEPRAZOLE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE SODIUM SALT; REGISTERED: UK 10555/0010 19980508; UK 10555/0008 19980508 |
| 0719278 | 03C0030 | France | ⤷ Try a Trial | PRODUCT NAME: DUTASTERIDE; NAT. REGISTRATION NO/DATE: NL 28 131 20030327; FIRST REGISTRATION: SE - 17 871 20020719 |
| 0719278 | SPC/GB03/018 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DUTASTERIDE, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SOLVATE.; REGISTERED: SE 17871 20020719; SE 17872 20020719; UK PL 19494/0005 20030117; UK PL 19494/0006 20030117 |
| 0719278 | 23/2003 | Austria | ⤷ Try a Trial | PRODUCT NAME: DUTASTERIDE, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SOLVATES; NAT. REGISTRATION NO/DATE: 1-24844, 1-24845 20030217; FIRST REGISTRATION: SE 17871, 17872 20020719 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.