Validus Pharms Company Profile
✉ Email this page to a colleague
What is the competitive landscape for VALIDUS PHARMS, and what generic alternatives to VALIDUS PHARMS drugs are available?
VALIDUS PHARMS has fourteen approved drugs.
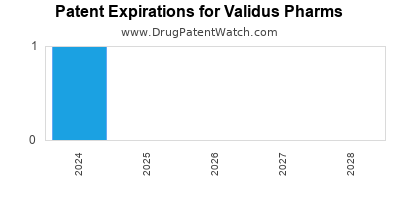
There is one US patent protecting VALIDUS PHARMS drugs.
There is one patent family member on VALIDUS PHARMS drugs in one country and ten supplementary protection certificates in six countries.
Summary for Validus Pharms
| International Patents: | 1 |
| US Patents: | 1 |
| Tradenames: | 12 |
| Ingredients: | 12 |
| NDAs: | 14 |
Drugs and US Patents for Validus Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Validus Pharms | LASIX | furosemide | TABLET;ORAL | 016273-003 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Validus Pharms | LASIX | furosemide | TABLET;ORAL | 016273-001 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Validus Pharms | DEMEROL | meperidine hydrochloride | INJECTABLE;INJECTION | 005010-007 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Validus Pharms | ANZEMET | dolasetron mesylate | INJECTABLE;INJECTION | 020624-003 | Dec 11, 2001 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Validus Pharms | ANZEMET | dolasetron mesylate | INJECTABLE;INJECTION | 020624-002 | Sep 11, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Validus Pharms | LOPRESSOR | metoprolol tartrate | TABLET;ORAL | 017963-001 | Approved Prior to Jan 1, 1982 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Validus Pharms
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Validus Pharms | TRENTAL | pentoxifylline | TABLET, EXTENDED RELEASE;ORAL | 018631-001 | Aug 30, 1984 | 3,737,433 | ⤷ Try a Trial |
| Validus Pharms | EQUETRO | carbamazepine | CAPSULE, EXTENDED RELEASE;ORAL | 021710-003 | Dec 10, 2004 | 5,326,570 | ⤷ Try a Trial |
| Validus Pharms | LOPRESSOR | metoprolol tartrate | TABLET;ORAL | 017963-001 | Approved Prior to Jan 1, 1982 | 3,998,790 | ⤷ Try a Trial |
| Validus Pharms | LOTENSIN | benazepril hydrochloride | TABLET;ORAL | 019851-001 | Jun 25, 1991 | 4,410,520*PED | ⤷ Try a Trial |
| Validus Pharms | LOTENSIN HCT | benazepril hydrochloride; hydrochlorothiazide | TABLET;ORAL | 020033-004 | May 19, 1992 | 4,410,520*PED | ⤷ Try a Trial |
| Validus Pharms | LOPRESSOR | metoprolol tartrate | TABLET;ORAL | 017963-001 | Approved Prior to Jan 1, 1982 | 3,876,802 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for VALIDUS PHARMS drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Capsules | 100 mg | ➤ Subscribe | 2014-05-23 |
| ➤ Subscribe | Extended-release Capsules | 200 mg and 300 mg | ➤ Subscribe | 2007-08-21 |
International Patents for Validus Pharms Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 2452588 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Validus Pharms Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0454511 | 99C0009 | Belgium | ⤷ Try a Trial | PRODUCT NAME: IRBESARTAN / HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/98/086/001 19981015 |
| 0503785 | CA 2011 00026 | Denmark | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF OLMESARTAN MEDOXOMIL, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, AND AMLODIPINE BESYLATE AND HYDROCHLOROTHIAZIDE; NAT. REG. NO/DATE: 46260-46269 (DK) 20110323; FIRST REG. NO/DATE: DE 79810.00.00 20101216 |
| 0502314 | SPC/GB02/037 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TELMISARTAN, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, AND HYDROCHLOROTHIAZIDE; REGISTERED: UK EU/1/02/213/001 20020419; UK EU/1/02/213/002 20020419; UK EU/1/02/213/003 20020419; UK EU/1/02/214/004 20020419; UK EU/1/02/213/005 20020419; UK EU/1/02/213/006 20020419; UK EU/1/02/213/007 20020419; UK EU/1/02/213/008 20020419; UK EU/1/02/213/009 20020419; UK EU/1/02/213/010 20020419 |
| 0565634 | 06C0030 | France | ⤷ Try a Trial | PRODUCT NAME: EPROSARTAN MESYLATE; HYDROCHLOROTHIAZIDE; NAT. REGISTRATION NO/DATE: NL 32075 20060623; FIRST REGISTRATION: LI - 55783 01 20020607 |
| 0502314 | C300095 | Netherlands | ⤷ Try a Trial | PRODCUT NAME: TELMISARTAN, DESGEWENST IN DE VORM VAN EEN FYSIOLOGISCH VERDRAAGBAAR ZOUT, EN HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/02/213/001-010 20020419 |
| 0266730 | C970043 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DOLASETRON, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AAN- VAARDBAAR ZUURADDITIEZOUT, IN HET BIJZONDER HET MESYLAAT; NAT REGISTRATION NO/DATE: RVG 21707 - RVG 21709 19970623; FIRST REGISTRATION: GB PL 04425/0150, PL 04425/0152, PL 04425/0154 19960926 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.