Teva Pharm Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TEVA PHARM, and when can generic versions of TEVA PHARM drugs launch?
TEVA PHARM has three hundred and twenty approved drugs.
There are thirty-two US patents protecting TEVA PHARM drugs. There are forty-four tentative approvals on TEVA PHARM drugs.
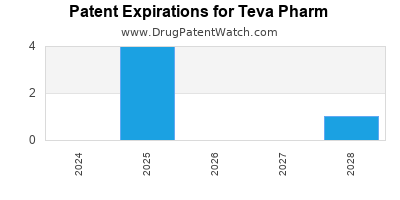
There are four hundred and thirty-six patent family members on TEVA PHARM drugs in thirty-six countries and nine hundred and sixteen supplementary protection certificates in eighteen countries.
Summary for Teva Pharm
| International Patents: | 436 |
| US Patents: | 32 |
| Tradenames: | 268 |
| Ingredients: | 260 |
| NDAs: | 320 |
| Patent Litigation for Teva Pharm: | See patent lawsuits for Teva Pharm |
| PTAB Cases with Teva Pharm as petitioner: | See PTAB cases with Teva Pharm as petitioner |
Drugs and US Patents for Teva Pharm
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Pharms Usa Inc | BREXPIPRAZOLE | brexpiprazole | TABLET;ORAL | 213692-002 | Aug 11, 2022 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms Usa | CARBOPLATIN | carboplatin | INJECTABLE;INTRAVENOUS | 077139-001 | Sep 21, 2005 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms | ATORVASTATIN CALCIUM | atorvastatin calcium | TABLET;ORAL | 078773-001 | May 29, 2012 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms Usa | ONSURA | ethinyl estradiol; norelgestromin | FILM, EXTENDED RELEASE;TRANSDERMAL | 213977-001 | Aug 25, 2021 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Pharms Usa | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | INJECTABLE;INJECTION | 209852-001 | Oct 5, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Pharms Usa | IVERMECTIN | ivermectin | LOTION;TOPICAL | 212485-001 | Mar 21, 2022 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Teva Pharms Usa | EPTIFIBATIDE | eptifibatide | INJECTABLE;INJECTION | 091555-001 | Jun 5, 2015 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Teva Pharm
Paragraph IV (Patent) Challenges for TEVA PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
| ➤ Subscribe | for Injection | 3.5 mg/vial | ➤ Subscribe | 2016-10-26 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-01-29 |
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | Extended-release Capsule | 15 mg and 30 mg | ➤ Subscribe | 2008-08-11 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Injection | 40 mg/mL, 1 mL pre-filled syringe | ➤ Subscribe | 2014-02-26 |
Premature patent expirations for TEVA PHARM
Expiration due to failure to pay maintenance fee
| Patent Number | Expiration Date |
|---|---|
| ⤷ Try a Trial | ⤷ Try a Trial |
International Patents for Teva Pharm Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Canada | 3187288 | ⤷ Try a Trial |
| Canada | 2799625 | ⤷ Try a Trial |
| Canada | 2540580 | ⤷ Try a Trial |
| Eurasian Patent Organization | 039125 | ⤷ Try a Trial |
| China | 106999681 | ⤷ Try a Trial |
| European Patent Office | 3501584 | ⤷ Try a Trial |
| European Patent Office | 3247331 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Teva Pharm Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3300601 | LUC00271 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINATION OF DAUNORUBICIN AND CYTARABINE; AUTHORISATION NUMBER AND DATE: EU/1/18/1308 20180827 |
| 2316456 | 17C1058 | France | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE NALTREXONE ET,BUPROPION OU SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER CHLORHYDRATE DE BUPROPION; REGISTRATION NO/DATE: EU/1/14/988 20150330 |
| 0398460 | 12/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON IN KOMBINATION MIT ESTRADIOL; NAT. REGISTRATION NO/DATE: 1-25178, 1-25179 20031127; FIRST REGISTRATION: NL RVG 27505 20021211 |
| 0810209 | 33/2007 | Austria | ⤷ Try a Trial | PRODUCT NAME: DARUNAVIR UND DESSEN PHARMAZEUTISCH VERTRAEGLICHE SALZE; REGISTRATION NO/DATE: EU/1/06/380/001 - EU/1/06/380/008 20070212 |
| 1613288 | 2011C/030 | Belgium | ⤷ Try a Trial | PRODUCT NAME: FINGOLIMOD; AUTHORISATION NUMBER AND DATE: EU/1/11/677/001 20110322 |
| 0961612 | 300417 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: PACLITAXEL ALBUMINE; REGISTRATION NO/DATE: EU/1/07/428/001 20080114 |
| 1663240 | 1590056-6 | Sweden | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF RILPIVIRINE, OR A PHARMACEUTICALLY ACCEPTABLE SALT OF RILPIVIRINE, INCLUDING THE HYDROCHLORIDE SALT OF RILPIVIRINE, AND TENOFOVIR DISOPROXIL, IN PARTICULAR TENOFOVIR DISOPROXIL FUMARATE; REG. NO/DATE: EU/1/11/737 20111128 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.