Specgx Llc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SPECGX LLC, and what generic alternatives to SPECGX LLC drugs are available?
SPECGX LLC has sixty-one approved drugs.
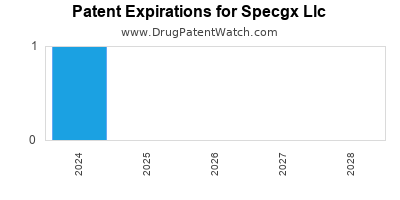
There is one US patent protecting SPECGX LLC drugs.
There are ten patent family members on SPECGX LLC drugs in nine countries and thirty supplementary protection certificates in ten countries.
Summary for Specgx Llc
| International Patents: | 10 |
| US Patents: | 1 |
| Tradenames: | 47 |
| Ingredients: | 31 |
| NDAs: | 61 |
| Drug Master File Entries: | 73 |
Drugs and US Patents for Specgx Llc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specgx Llc | FENTANYL-62 | fentanyl | FILM, EXTENDED RELEASE;TRANSDERMAL | 077154-007 | Jan 14, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Specgx Llc | ANEXSIA 5/325 | acetaminophen; hydrocodone bitartrate | TABLET;ORAL | 040409-001 | Oct 20, 2000 | AA | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Specgx Llc | ROXICODONE | oxycodone hydrochloride | TABLET;ORAL | 021011-002 | Aug 31, 2000 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Specgx Llc | FLUOXETINE HYDROCHLORIDE | fluoxetine hydrochloride | SOLUTION;ORAL | 075920-001 | Jan 29, 2002 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Specgx Llc | OXYCODONE HYDROCHLORIDE | oxycodone hydrochloride | TABLET;ORAL | 076758-003 | Mar 19, 2007 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Specgx Llc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Specgx Llc | EXALGO | hydromorphone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021217-003 | Mar 1, 2010 | 5,702,725 | ⤷ Try a Trial |
| Specgx Llc | RESTORIL | temazepam | CAPSULE;ORAL | 018163-003 | Oct 25, 1991 | 5,030,632 | ⤷ Try a Trial |
| Specgx Llc | EXALGO | hydromorphone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021217-004 | Aug 24, 2012 | 5,702,725 | ⤷ Try a Trial |
| Specgx Llc | PAMELOR | nortriptyline hydrochloride | CAPSULE;ORAL | 018013-004 | Approved Prior to Jan 1, 1982 | 3,922,305 | ⤷ Try a Trial |
| Specgx Llc | EXALGO | hydromorphone hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 021217-004 | Aug 24, 2012 | 5,914,131 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SPECGX LLC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Oral Solution | 5 mg/5 mL and 10 mg/5 mL | ➤ Subscribe | 2010-04-13 |
International Patents for Specgx Llc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 1680144 | ⤷ Try a Trial |
| China | 1867359 | ⤷ Try a Trial |
| Canada | 2540052 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005035000 | ⤷ Try a Trial |
| Spain | 2373042 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Specgx Llc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2316456 | 1790064-8 | Sweden | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR NALTREXONE HYDROCHLORIDE, AND BUPROPION OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BUPROPION HYDROCHLORIDE; REG. NO/DATE: EU/1/14/988 20150330 |
| 2316456 | 2017C/064 | Belgium | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE/BUPROPION; AUTHORISATION NUMBER AND DATE: EU/1/14/988 20150330 |
| 1635783 | C300653 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ELKE DOOR HET BASISOCTROOI BESCHERMDE VERSCHIJNINGSVORM; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100906 |
| 1769785 | C300521 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL; REG NO/DATE: EU/2/11/127/001 20111006 |
| 0901368 | C300523 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL; REGISTRATION NO/DATE: EU/2/11/127/001 20111006 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.