Novo Nordisk Inc Company Profile
✉ Email this page to a colleague
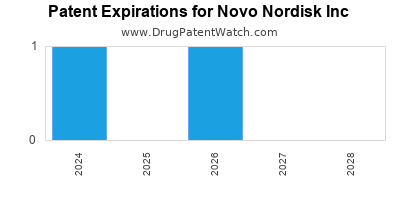
What is the competitive landscape for NOVO NORDISK INC, and when can generic versions of NOVO NORDISK INC drugs launch?
NOVO NORDISK INC has four approved drugs.
There are four US patents protecting NOVO NORDISK INC drugs.
There are eighty-three patent family members on NOVO NORDISK INC drugs in twenty-nine countries and one hundred supplementary protection certificates in sixteen countries.
Summary for Novo Nordisk Inc
| International Patents: | 83 |
| US Patents: | 4 |
| Tradenames: | 4 |
| Ingredients: | 3 |
| NDAs: | 4 |
| Drug Master File Entries: | 3 |
Drugs and US Patents for Novo Nordisk Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | 9,968,659*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo Nordisk Inc | INNOFEM | estradiol | TABLET;ORAL | 040312-001 | Nov 19, 1999 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | 9,265,893*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo Nordisk Inc | PRANDIMET | metformin hydrochloride; repaglinide | TABLET;ORAL | 022386-001 | Jun 23, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novo Nordisk Inc | PRANDIMET | metformin hydrochloride; repaglinide | TABLET;ORAL | 022386-002 | Jun 23, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novo Nordisk Inc | INNOFEM | estradiol | TABLET;ORAL | 040312-003 | Nov 19, 1999 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Novo Nordisk Inc | VICTOZA | liraglutide recombinant | SOLUTION;SUBCUTANEOUS | 022341-001 | Jan 25, 2010 | RX | Yes | Yes | 7,762,994*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Novo Nordisk Inc
Paragraph IV (Patent) Challenges for NOVO NORDISK INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 1 mg/500 mg and 2 mg/500 mg | ➤ Subscribe | 2009-04-09 |
| ➤ Subscribe | Injection | 18 mg/3 mL prefilled syringe | ➤ Subscribe | 2016-12-12 |
| ➤ Subscribe | Vaginal Tablets | 10 mcg | ➤ Subscribe | 2013-01-02 |
International Patents for Novo Nordisk Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2003243922 | ⤷ Try a Trial |
| Canada | 2491356 | ⤷ Try a Trial |
| Hong Kong | 1246683 | ⤷ Try a Trial |
| Philippines | 12018501844 | ⤷ Try a Trial |
| Poland | 2109474 | ⤷ Try a Trial |
| Spain | 2660320 | ⤷ Try a Trial |
| Australia | 2004290862 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Novo Nordisk Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| 0584952 | 99C0004 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL, HEMIHYDRATE, NORETHISTERONE, ACETATE; NAT. REGISTRATION NO/DATE: NL 23753 19981210; FIRST REGISTRATION: SE - 14 007 19980306 |
| 1412357 | 50/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTIN, GEGEBENENFALLS IN DER FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, INSBESONDERE ALS MONOPHOSPHAT, UND METFORMIN, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES, INSBESONDERE DES HYDROCHLORIDS; NAT. REGISTRATION NO/DATE: EU/1/08/455/001-014, EU/1/08/456/001-014, EU/1/08/457/001-014 20080716; FIRST REGISTRATION: CH 58450 01-03 20080408 |
| 1412357 | C300357 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SITAGLIPTINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVA ARDBAAR ZOUT, IN HET BIJZONDER HET MONOFOSFAAT, EN METFORMINE DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER HET HYDROCHLORIDE; REGISTRATION NO/DATE: EU/1/08/455/001-014 20080716 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 2498758 | 301040 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: METFORMINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; SAXAGLIPTINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR SOLVAAT DAARVAN; REGISTRATION NO/DATE: EU/1/19/1401 20191113 |
| 1506211 | PA2014026,C1506211 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINUM + METFORMINUM; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.