Gilead Sciences Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for GILEAD SCIENCES INC, and what generic alternatives to GILEAD SCIENCES INC drugs are available?
GILEAD SCIENCES INC has twenty-three approved drugs.
There are eighty-two US patents protecting GILEAD SCIENCES INC drugs.
There are two thousand two hundred and twenty-eight patent family members on GILEAD SCIENCES INC drugs in sixty-six countries and three hundred and seventy supplementary protection certificates in nineteen countries.
Summary for Gilead Sciences Inc
| International Patents: | 2228 |
| US Patents: | 82 |
| Tradenames: | 18 |
| Ingredients: | 18 |
| NDAs: | 23 |
| Drug Master File Entries: | 7 |
| Patent Litigation for Gilead Sciences Inc: | See patent lawsuits for Gilead Sciences Inc |
Drugs and US Patents for Gilead Sciences Inc
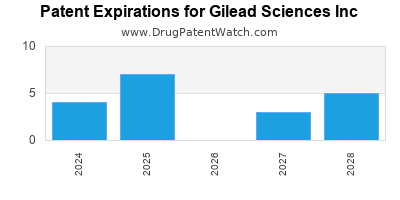
Expired US Patents for Gilead Sciences Inc
Paragraph IV (Patent) Challenges for GILEAD SCIENCES INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2015-12-09 |
| ➤ Subscribe | Tablets | 150 mg, 200 mg, and 250 mg | ➤ Subscribe | 2012-05-17 |
| ➤ Subscribe | Tablets | 150 mg, 150 mg, 200 mg, 300 mg | ➤ Subscribe | 2018-10-04 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2010-01-26 |
| ➤ Subscribe | Tablets | 200 mg/25 mg/300 mg | ➤ Subscribe | 2015-05-20 |
International Patents for Gilead Sciences Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2017273851 | ⤷ Try a Trial |
| Hong Kong | 1164737 | ⤷ Try a Trial |
| South Africa | 201200310 | ⤷ Try a Trial |
| New Zealand | 709926 | ⤷ Try a Trial |
| Norway | 2717902 | ⤷ Try a Trial |
| South Korea | 100665919 | ⤷ Try a Trial |
| European Patent Office | 3025718 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Gilead Sciences Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1419152 | 122012000035 | Germany | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRIN UND DESSEN STEREOCHEMISCH ISOMERE FORMEN, SOWIE DESSEN PHARMAZEUTISCH UNBEDENKLICHE ADDITIONSSALZE, EINSCHLIESSLICH DES CHLORWASSERSTOFFSAEURESALZES VON RILPIVIRIN; REGISTRATION NO/DATE: EU/1/11/736/001 20111128 |
| 1632232 | CA 2016 00065 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EN KOMBINATION AF: RILPIVIRINHYDROCHLORID ELLER EN TERAPEUTISK AEKVIVALENT FORM DERAF SOM ER BESKYTTET AF GRUNDPATENTET, OG TENOFOVIRALAFENAMID, ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT TENOFOVIRALAFENAMIDFUMARAT; REG. NO/DATE: EU/1/16/1112/001 20160623 |
| 2430014 | SPC/GB16/008 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LEDIPASVIR; REGISTERED: UK EU/1/14/958(001-002) 20141118 |
| 1663240 | 132016000024751 | Italy | ⤷ Try a Trial | PRODUCT NAME: ASSOCIAZIONE DI RILPIVIRINA E OGNI SUA FORMA TERAPEUTICAMENTE EQUIVALENTE PROTETTA DAL BREVETTO DI BASE, COME SALI DI ADDIZIONE FARMACEUTICAMENTE ACCETTABILI DI RILPIVIRINA, COMPRESO IL SUO SALE CLORIDRATO E EMTRICITABINA(EVIPLERA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/11/737/001-002, 20111128 |
| 3808743 | C03808743/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: RILPIVIRIN UND EMTRICITABIN; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 62155 12.03.2013 |
| 1761540 | PA2017004,C1761540 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: IDELALISIBAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; REGISTRATION NO/DATE: EU/1/14/938 20140918 |
| 2822954 | LUC00083 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF, IN PARTICULAR BICTEGRAVIR SODIUM; AUTHORISATION NUMBER AND DATE: EU/1/18/1289 20180625 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.