Cubist Pharms Llc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for CUBIST PHARMS LLC, and what generic alternatives to CUBIST PHARMS LLC drugs are available?
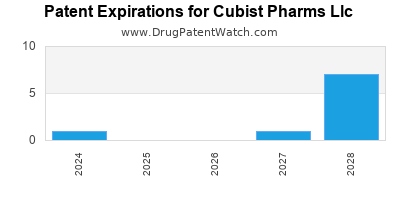
CUBIST PHARMS LLC has six approved drugs.
There are twenty-nine US patents protecting CUBIST PHARMS LLC drugs.
There are three hundred and forty-one patent family members on CUBIST PHARMS LLC drugs in fifty-three countries and fifty-five supplementary protection certificates in eighteen countries.
Summary for Cubist Pharms Llc
| International Patents: | 341 |
| US Patents: | 29 |
| Tradenames: | 5 |
| Ingredients: | 4 |
| NDAs: | 6 |
Drugs and US Patents for Cubist Pharms Llc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cubist Pharms Llc | DIFICID | fidaxomicin | FOR SUSPENSION;ORAL | 213138-001 | Jan 24, 2020 | RX | Yes | Yes | 7,906,489*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cubist Pharms Llc | DIFICID | fidaxomicin | FOR SUSPENSION;ORAL | 213138-001 | Jan 24, 2020 | RX | Yes | Yes | 8,859,510*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Cubist Pharms Llc | SIVEXTRO | tedizolid phosphate | POWDER;INTRAVENOUS | 205436-001 | Jun 20, 2014 | RX | Yes | Yes | 9,988,406 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Cubist Pharms Llc | ZERBAXA | ceftolozane sulfate; tazobactam sodium | POWDER;INTRAVENOUS | 206829-001 | Dec 19, 2014 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Cubist Pharms Llc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Cubist Pharms Llc | CUBICIN | daptomycin | POWDER;INTRAVENOUS | 021572-002 | Sep 12, 2003 | 8,058,238 | ⤷ Try a Trial |
| Cubist Pharms Llc | CUBICIN | daptomycin | POWDER;INTRAVENOUS | 021572-002 | Sep 12, 2003 | 6,468,967 | ⤷ Try a Trial |
| Cubist Pharms Llc | CUBICIN | daptomycin | POWDER;INTRAVENOUS | 021572-001 | Sep 12, 2003 | 6,852,689 | ⤷ Try a Trial |
| Cubist Pharms Llc | CUBICIN | daptomycin | POWDER;INTRAVENOUS | 021572-001 | Sep 12, 2003 | 8,058,238 | ⤷ Try a Trial |
| Cubist Pharms Llc | CUBICIN | daptomycin | POWDER;INTRAVENOUS | 021572-001 | Sep 12, 2003 | 5,912,226 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for CUBIST PHARMS LLC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | For Injection | 500 mg/vial | ➤ Subscribe | 2008-11-19 |
| ➤ Subscribe | Tablets | 200 mg | ➤ Subscribe | 2015-05-27 |
International Patents for Cubist Pharms Llc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Australia | 2004299413 | ⤷ Try a Trial |
| Eurasian Patent Organization | 028342 | ⤷ Try a Trial |
| Russian Federation | 2478643 | ⤷ Try a Trial |
| European Patent Office | 2753326 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005058886 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Cubist Pharms Llc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1115417 | 06C0022 | France | ⤷ Try a Trial | PRODUCT NAME: DAPTOMYCINE; REGISTRATION NO/DATE: EU/1/05/328/001-002 20060119 |
| 1115417 | SZ 22/2006 | Austria | ⤷ Try a Trial | PRODUCT NAME: DAPTOMYCIN |
| 1556389 | 602 | Finland | ⤷ Try a Trial | |
| 1556389 | CR 2016 00004 | Denmark | ⤷ Try a Trial | PRODUCT NAME: CEFTOLOZAN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT ET SVOVLSYRESALT; REG. NO/DATE: EU/1/15/1032/001 20150922 |
| 1539977 | C201530022 | Spain | ⤷ Try a Trial | PRODUCT NAME: FIDAXOMICINA; NATIONAL AUTHORISATION NUMBER: EU/1/11/733/001-004; DATE OF AUTHORISATION: 20111207; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/11/733/001-004; DATE OF FIRST AUTHORISATION IN EEA: 20111207 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.