Bausch And Lomb Inc Company Profile
✉ Email this page to a colleague
What is the competitive landscape for BAUSCH AND LOMB INC, and when can generic versions of BAUSCH AND LOMB INC drugs launch?
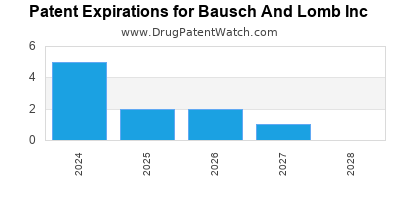
BAUSCH AND LOMB INC has nineteen approved drugs.
There are thirty-two US patents protecting BAUSCH AND LOMB INC drugs.
There are three hundred and seventy-four patent family members on BAUSCH AND LOMB INC drugs in thirty-one countries and forty-one supplementary protection certificates in fourteen countries.
Summary for Bausch And Lomb Inc
| International Patents: | 374 |
| US Patents: | 32 |
| Tradenames: | 19 |
| Ingredients: | 15 |
| NDAs: | 19 |
| Drug Master File Entries: | 1 |
Drugs and US Patents for Bausch And Lomb Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | LOTEMAX SM | loteprednol etabonate | GEL;OPHTHALMIC | 208219-001 | Feb 22, 2019 | RX | Yes | Yes | 10,596,107 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bausch And Lomb Inc | LACRISERT | hydroxypropyl cellulose | INSERT;OPHTHALMIC | 018771-001 | Approved Prior to Jan 1, 1982 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bausch And Lomb Inc | XIPERE | triamcinolone acetonide | SUSPENSION;INJECTION | 211950-001 | Oct 22, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Bausch And Lomb Inc | LUMIFY | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 208144-001 | Dec 22, 2017 | OTC | Yes | Yes | 11,833,245 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Bausch And Lomb Inc
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch And Lomb Inc | XIBROM | bromfenac sodium | SOLUTION/DROPS;OPHTHALMIC | 021664-001 | Mar 24, 2005 | 4,910,225 | ⤷ Try a Trial |
| Bausch And Lomb Inc | TIMOPTIC-XE | timolol maleate | SOLUTION, GEL FORMING/DROPS;OPHTHALMIC | 020330-001 | Nov 4, 1993 | 4,195,085 | ⤷ Try a Trial |
| Bausch And Lomb Inc | MACUGEN | pegaptanib sodium | INJECTABLE;INTRAVITREAL | 021756-001 | Dec 17, 2004 | 6,147,204 | ⤷ Try a Trial |
| Bausch And Lomb Inc | MACUGEN | pegaptanib sodium | INJECTABLE;INTRAVITREAL | 021756-001 | Dec 17, 2004 | 5,932,462 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for BAUSCH AND LOMB INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Ophthalmic Solution | 1.5% | ➤ Subscribe | 2013-09-09 |
International Patents for Bausch And Lomb Inc Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 101873797 | ⤷ Try a Trial |
| Australia | 2013314370 | ⤷ Try a Trial |
| Japan | 2014221808 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2015050670 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Bausch And Lomb Inc Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0957929 | PA2006004,C0957929 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PEGAPTANIBUM; REGISTRATION NO/DATE: EU/1/05/325/001 20060131 |
| 0957929 | C00957929/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: PEGAPTANIB; REGISTRATION NUMBER/DATE: SWISSMEDIC 57459 15.02.2006 |
| 0509752 | 49/1999 | Austria | ⤷ Try a Trial | PRODUCT NAME: DORZOLAMID ODER EIN OPHTHALMOLOGISCH ANNEHMBARES SALZ DAVON, VORZUGSWEISE DORZOLAMIDHYDROCHLORID, UND TIMOLOL ODER EIN OPHTHALMOLOGISCH ANNEHMBARES SALZ DAVON, VORZUGSWEISE TIMOLOLMALEAT; NAT. REGISTRATION NO/DATE: 1-22701, 1-22702 19980828; FIRST REGISTRATION: DK 9794 19980306 |
| 1586316 | 132011901975261 | Italy | ⤷ Try a Trial | PRODUCT NAME: BROMFENAC(YELLOX); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/11/692/001, 20110518 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.