Apil Company Profile
✉ Email this page to a colleague
What is the competitive landscape for APIL, and when can generic versions of APIL drugs launch?
APIL has sixteen approved drugs.
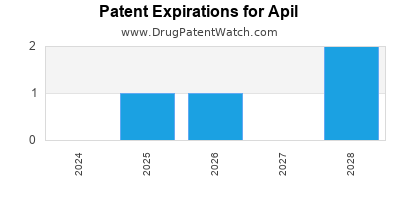
There are five US patents protecting APIL drugs.
There are ninety-six patent family members on APIL drugs in thirty-three countries and forty supplementary protection certificates in fourteen countries.
Drugs and US Patents for Apil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apil | NORETHINDRONE AND ETHINYL ESTRADIOL AND FERROUS FUMARATE | ethinyl estradiol; norethindrone | TABLET, CHEWABLE;ORAL | 022573-001 | Dec 22, 2010 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-004 | Apr 16, 2007 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Apil | SARAFEM | fluoxetine hydrochloride | TABLET;ORAL | 021860-002 | May 19, 2006 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Apil | ATELVIA | risedronate sodium | TABLET, DELAYED RELEASE;ORAL | 022560-001 | Oct 8, 2010 | AB | RX | Yes | Yes | 8,246,989 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Apil | FEMHRT | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 021065-002 | Oct 15, 1999 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Apil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Apil | NORETHINDRONE AND ETHINYL ESTRADIOL AND FERROUS FUMARATE | ethinyl estradiol; norethindrone | TABLET, CHEWABLE;ORAL | 022573-001 | Dec 22, 2010 | 5,552,394 | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-003 | May 25, 2002 | 6,165,513*PED | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-005 | Apr 22, 2008 | 7,718,634*PED | ⤷ Try a Trial |
| Apil | DIDRONEL | etidronate disodium | TABLET;ORAL | 017831-002 | Approved Prior to Jan 1, 1982 | 3,683,080 | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-003 | May 25, 2002 | 6,096,342*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for APIL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Chewable Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2014-04-23 |
| ➤ Subscribe | Delayed-release Tablets | 35 mg | ➤ Subscribe | 2011-07-19 |
| ➤ Subscribe | Tablets | 75 mg | ➤ Subscribe | 2007-09-10 |
| ➤ Subscribe | Chewable Tablets | 0.4 mg/0.035 mg | ➤ Subscribe | 2007-04-27 |
| ➤ Subscribe | Delayed-release Tablets | 400 mg | ➤ Subscribe | 2007-06-22 |
| ➤ Subscribe | Tablets | 5 mg, 30 mg and 35 mg | ➤ Subscribe | 2004-04-23 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2008-08-12 |
International Patents for Apil Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 2305266 | ⤷ Try a Trial |
| Japan | 2008535912 | ⤷ Try a Trial |
| South Korea | 101411117 | ⤷ Try a Trial |
| Taiwan | 200607514 | ⤷ Try a Trial |
| Norway | 20042565 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Apil Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| 1380301 | CA 2009 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETADEXCLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 42417 (DK) 20080619; FIRST REG. NO/DATE: NL 33842 20070629 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0398460 | C300221 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| 0770388 | 2009/012 | Ireland | ⤷ Try a Trial | PRODUCT NAME: QLAIRA-ESTRADIOL VALERATE/DIENOGEST; NAT REGISTRATION NO/DATE: PA1410/58/1 20090109; FIRST REGISTRATION NO/DATE: BE327792 20081103 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.