Agile Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AGILE, and when can generic versions of AGILE drugs launch?
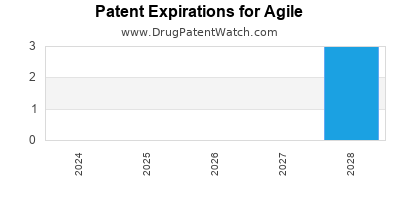
AGILE has one approved drug.
There are three US patents protecting AGILE drugs.
There are nineteen patent family members on AGILE drugs in twelve countries and fifteen supplementary protection certificates in eight countries.
Drugs and US Patents for Agile
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | 8,747,888 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | 9,050,348 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | RX | Yes | Yes | 8,246,978 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Agile
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | 8,883,196 | ⤷ Try a Trial |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | 8,221,784 | ⤷ Try a Trial |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | 7,045,145 | ⤷ Try a Trial |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | 8,221,785 | ⤷ Try a Trial |
| Agile | TWIRLA | ethinyl estradiol; levonorgestrel | SYSTEM;TRANSDERMAL | 204017-001 | Feb 14, 2020 | 7,384,650 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Agile Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2011088072 | ⤷ Try a Trial |
| Eurasian Patent Organization | 201070123 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2009009651 | ⤷ Try a Trial |
| Japan | 5603235 | ⤷ Try a Trial |
| Hong Kong | 1142798 | ⤷ Try a Trial |
| New Zealand | 582565 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Agile Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1453521 | 39/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL UND EINE KOMBINATION VON LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 136021 20150224; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 0771217 | 07C0001 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 1214076 | SZ 49/2008 | Austria | ⤷ Try a Trial | PRODUCT NAME: WIRKSTOFFKOMBINATION VON ETHINYLESTRADIOL UND DROSPIRENON |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.