Accord Company Profile
✉ Email this page to a colleague
What is the competitive landscape for ACCORD, and what generic alternatives to ACCORD drugs are available?
ACCORD has one hundred and fifty-five approved drugs.
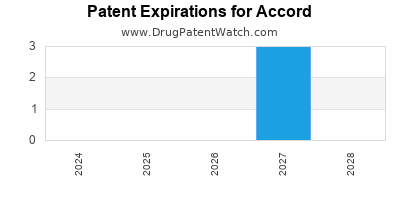
There are four US patents protecting ACCORD drugs. There are three tentative approvals on ACCORD drugs.
There are thirty-five patent family members on ACCORD drugs in eighteen countries and three hundred and eighty-six supplementary protection certificates in eighteen countries.
Summary for Accord
| International Patents: | 35 |
| US Patents: | 4 |
| Tradenames: | 132 |
| Ingredients: | 129 |
| NDAs: | 155 |
| Patent Litigation for Accord: | See patent lawsuits for Accord |
| PTAB Cases with Accord as petitioner: | See PTAB cases with Accord as petitioner |
Drugs and US Patents for Accord
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accord Hlthcare | ARGATROBAN IN SODIUM CHLORIDE | argatroban | SOLUTION;INTRAVENOUS | 212035-001 | Jun 7, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Accord Hlthcare | RANOLAZINE | ranolazine | TABLET, EXTENDED RELEASE;ORAL | 212930-002 | May 18, 2021 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Accord Hlthcare | TRAZODONE HYDROCHLORIDE | trazodone hydrochloride | TABLET;ORAL | 206923-002 | Sep 8, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Accord Hlthcare | DEXMEDETOMIDINE HYDROCHLORIDE | dexmedetomidine hydrochloride | INJECTABLE;INJECTION | 204023-001 | Feb 9, 2016 | AP | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for ACCORD drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | for Injection | 100 mcg/vial and 500 mcg/vial | ➤ Subscribe | 2015-04-14 |
| ➤ Subscribe | for Injection | 200 mcg/vial | ➤ Subscribe | 2015-05-01 |
International Patents for Accord Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| China | 111511385 | ⤷ Try a Trial |
| Russian Federation | 2756514 | ⤷ Try a Trial |
| Brazil | PI0706558 | ⤷ Try a Trial |
| Japan | 7223021 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Accord Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0253738 | C960002 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DOCETAXEL, DESGEWENST IN DE VORM VAN EEN TRIHYDRAAT; REGISTRATION NO/DATE: EU/1/95/002/001 - EU/1/95/002/002 19951127 |
| 0740668 | 03C0017 | France | ⤷ Try a Trial | PRODUCT NAME: TADALAFIL; REGISTRATION NO/DATE: EU/1/02/237/001-004 20021112 |
| 2435025 | 19C1040 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET DE FORMOTEROL (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); NAT. REGISTRATION NO/DATE: EU/1/18/1339 20181220; FIRST REGISTRATION: - EU/1/18/1339 20181220 |
| 0300652 | SPC/GB03/005 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: D-MEDETOMIDINE (4-((1S)-1-(2,3-DIMETHYLPHENYL)ETHYL)-1H-IMIDAZOLE) AND ITS NON-TOXIC PHARMACEUTICALLY ACCEPTABLE ACID ADDITION SALTS, ESPECIALLY THE HYDROCHLORIDE; REGISTERED: UK EU/2/02/033/001 20020830 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.