Mylan Speciality Lp Company Profile
✉ Email this page to a colleague
What is the competitive landscape for MYLAN SPECIALITY LP, and when can generic versions of MYLAN SPECIALITY LP drugs launch?
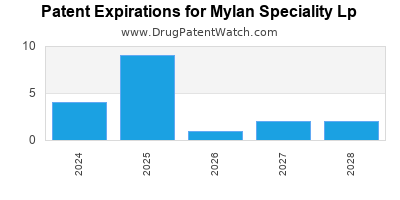
MYLAN SPECIALITY LP has thirty-one approved drugs.
There are twenty US patents protecting MYLAN SPECIALITY LP drugs.
There are one hundred and eighty-one patent family members on MYLAN SPECIALITY LP drugs in thirty-seven countries and seventy supplementary protection certificates in seventeen countries.
Summary for Mylan Speciality Lp
| International Patents: | 181 |
| US Patents: | 20 |
| Tradenames: | 35 |
| Ingredients: | 26 |
| NDAs: | 31 |
Drugs and US Patents for Mylan Speciality Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mylan Speciality Lp | AVC | sulfanilamide | CREAM;VAGINAL | 006530-003 | Jan 27, 1987 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | RX | Yes | Yes | 10,207,066 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mylan Speciality Lp | LEVALBUTEROL HYDROCHLORIDE | levalbuterol hydrochloride | SOLUTION;INHALATION | 077800-002 | Mar 15, 2013 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | RX | Yes | Yes | RE47526 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mylan Speciality Lp | COLYTE | polyethylene glycol 3350; potassium chloride; sodium bicarbonate; sodium chloride; sodium sulfate anhydrous | FOR SOLUTION;ORAL | 018983-006 | Oct 26, 1984 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mylan Speciality Lp | MUSE | alprostadil | SUPPOSITORY;URETHRAL | 020700-003 | Nov 19, 1996 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Mylan Speciality Lp | EPIPEN | epinephrine | INJECTABLE;INTRAMUSCULAR, SUBCUTANEOUS | 019430-001 | Dec 22, 1987 | AB | RX | Yes | Yes | 7,449,012 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Mylan Speciality Lp
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mylan Speciality Lp | MUSE | alprostadil | SUPPOSITORY;URETHRAL | 020700-002 | Nov 19, 1996 | 5,242,391 | ⤷ Try a Trial |
| Mylan Speciality Lp | DEMADEX | torsemide | TABLET;ORAL | 020136-003 | Aug 23, 1993 | 4,822,807 | ⤷ Try a Trial |
| Mylan Speciality Lp | TOBI PODHALER | tobramycin | POWDER;INHALATION | 201688-001 | Mar 22, 2013 | 7,097,827 | ⤷ Try a Trial |
| Mylan Speciality Lp | EDLUAR | zolpidem tartrate | TABLET;SUBLINGUAL | 021997-001 | Mar 13, 2009 | 6,761,910 | ⤷ Try a Trial |
| Mylan Speciality Lp | MUSE | alprostadil | SUPPOSITORY;URETHRAL | 020700-001 | Nov 19, 1996 | 4,801,587 | ⤷ Try a Trial |
| Mylan Speciality Lp | EDLUAR | zolpidem tartrate | TABLET;SUBLINGUAL | 021997-001 | Mar 13, 2009 | 8,512,747 | ⤷ Try a Trial |
| Mylan Speciality Lp | MUSE | alprostadil | SUPPOSITORY;URETHRAL | 020700-001 | Nov 19, 1996 | 5,242,391 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MYLAN SPECIALITY LP drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection (Auto-injector) | 0.15 mg/0.3 mL and 0.3 mg/0.3 mL | ➤ Subscribe | 2008-11-21 |
| ➤ Subscribe | Inhalation Solution | 0.021% and 0.042% | ➤ Subscribe | 2005-10-19 |
| ➤ Subscribe | Nasal Spray | 205.5 mcg/spray | ➤ Subscribe | 2011-12-15 |
| ➤ Subscribe | Sublingual Tablets | 5 mg and 10 mg | ➤ Subscribe | 2010-04-29 |
| ➤ Subscribe | Injection (Auto-injector) | 0.15 mg/0.3 mL and 0.3 mg/0.3 mL | ➤ Subscribe | 2008-11-21 |
| ➤ Subscribe | Ophthalmic Solution | 0.05% | ➤ Subscribe | 2006-12-13 |
| ➤ Subscribe | Inhalation Solution | 300 mg/5 mL | ➤ Subscribe | 2009-06-29 |
| ➤ Subscribe | Nasal Spray | 137 mcg/50 mcg per spray | ➤ Subscribe | 2014-06-13 |
International Patents for Mylan Speciality Lp Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Israel | 238308 | ⤷ Try a Trial |
| Cyprus | 1115386 | ⤷ Try a Trial |
| United Kingdom | 0423800 | ⤷ Try a Trial |
| Poland | 2340872 | ⤷ Try a Trial |
| Canada | 2576776 | ⤷ Try a Trial |
| Tunisia | SN04247 | ⤷ Try a Trial |
| Slovenia | 1786491 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Mylan Speciality Lp Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0398460 | SPC/GB04/032 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL, OPTIONALLY IN THE FORM OF A HYDRATE, TOGETHER WITH DROSPIRENONE; REGISTERED: NL RVG 27505 20021211; UK PL 00053/0341 20040310 |
| 0770388 | 2009/012 | Ireland | ⤷ Try a Trial | PRODUCT NAME: QLAIRA-ESTRADIOL VALERATE/DIENOGEST; NAT REGISTRATION NO/DATE: PA1410/58/1 20090109; FIRST REGISTRATION NO/DATE: BE327792 20081103 |
| 1280520 | 14/2015 | Austria | ⤷ Try a Trial | PRODUCT NAME: TOBRAMYCIN ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/10/652/001-003 20110720 |
| 0402407 | 97C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL HEMIHYDRAAT; NAT. REGISTRATION NO/DATE: 298 IS 190 F 15 19960806; FIRST REGISTRATION: GB PL/0053/0241 19950711 |
| 1519731 | 1390033-7 | Sweden | ⤷ Try a Trial | PRODUCT NAME: AZELASTIN, ELLER ETT FARMACEUTISKT GODTAGBART SALT DAERAV, OCH EN FARMACEUTISKT GODTAGBAR ESTER AV FLUTIKASON; NAT. REG. NO/DATE: MTNR 47084 20130221; FIRST REG.: SK 24/0055/13-S 20130215 |
| 1453521 | 132016000025143 | Italy | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ED ETINILESTRADIOLO(SEASONIQUE); AUTHORISATION NUMBER(S) AND DATE(S): 17/0017/15-S, 20150211;042139016, 20150414 |
| 1214076 | C01214076/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE + ETHINYLESTRADIOL; REGISTRATION NUMBER/DATE: SWISSMEDIC 57946 13.06.2008 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.