Apil Company Profile
✉ Email this page to a colleague
What is the competitive landscape for APIL, and when can generic versions of APIL drugs launch?
APIL has fifteen approved drugs.
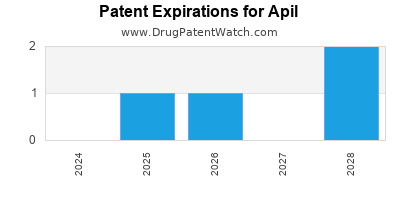
There are five US patents protecting APIL drugs.
There are ninety-six patent family members on APIL drugs in thirty-three countries and forty supplementary protection certificates in fourteen countries.
Drugs and US Patents for Apil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-002 | Apr 14, 2000 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Apil | FEMCON FE | ethinyl estradiol; norethindrone | TABLET;ORAL | 021490-001 | Nov 14, 2003 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Apil | LO LOESTRIN FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 022501-001 | Oct 21, 2010 | RX | Yes | Yes | 7,704,984 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Apil | ATELVIA | risedronate sodium | TABLET, DELAYED RELEASE;ORAL | 022560-001 | Oct 8, 2010 | AB | RX | Yes | Yes | 8,246,989 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-004 | Apr 16, 2007 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Apil | FEMTRACE | estradiol acetate | TABLET;ORAL | 021633-003 | Aug 20, 2004 | DISCN | Yes | No | 7,572,779 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Apil | MINASTRIN 24 FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL | 203667-001 | May 8, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Apil
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Apil | ESTROSTEP FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL-28 | 020130-002 | Oct 9, 1996 | 5,010,070 | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-001 | Mar 27, 1998 | 5,994,329 | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-004 | Apr 16, 2007 | 6,165,513*PED | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-002 | Apr 14, 2000 | 5,994,329 | ⤷ Try a Trial |
| Apil | ESTROSTEP FE | ethinyl estradiol; norethindrone acetate | TABLET;ORAL-28 | 020130-002 | Oct 9, 1996 | 4,962,098 | ⤷ Try a Trial |
| Apil | ACTONEL | risedronate sodium | TABLET;ORAL | 020835-003 | May 25, 2002 | 6,096,342*PED | ⤷ Try a Trial |
| Apil | LO MINASTRIN FE | ethinyl estradiol; norethindrone acetate | TABLET, CHEWABLE, TABLET;ORAL | 204654-001 | Jul 24, 2013 | 5,552,394 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for APIL drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 400 mg | ➤ Subscribe | 2007-06-22 |
| ➤ Subscribe | Tablets | 5 mg, 30 mg and 35 mg | ➤ Subscribe | 2004-04-23 |
| ➤ Subscribe | Tablets | 150 mg | ➤ Subscribe | 2008-08-12 |
| ➤ Subscribe | Chewable Tablets | 1 mg/0.02 mg and 75 mg | ➤ Subscribe | 2014-04-23 |
| ➤ Subscribe | Delayed-release Tablets | 35 mg | ➤ Subscribe | 2011-07-19 |
| ➤ Subscribe | Tablets | 75 mg | ➤ Subscribe | 2007-09-10 |
| ➤ Subscribe | Chewable Tablets | 0.4 mg/0.035 mg | ➤ Subscribe | 2007-04-27 |
International Patents for Apil Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Israel | 160797 | ⤷ Try a Trial |
| China | 1956706 | ⤷ Try a Trial |
| China | 104248639 | ⤷ Try a Trial |
| South Africa | 200609446 | ⤷ Try a Trial |
| Germany | 602005022578 | ⤷ Try a Trial |
| Serbia | 51562 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005115331 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Apil Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0398460 | C300221 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENON EN ETHINYLESTRADIOL; REGISTRATION NO/DATE: RVG 23827 20000307 |
| 1453521 | C 2015 029 | Romania | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL SI ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: RO 7793/2015/001; DATE OF NATIONAL AUTHORISATION: 20150612; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): SK. 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150129 |
| 2782584 | 2021C/558 | Belgium | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17--ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210406 |
| 0770388 | PA2009004,C0770388 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOLI VALERAS + DIENOGESTUM; NAT. REGISTRATION NO/DATE: LT/1/09/1512/001, 2009 04 06 LT/1/09/1512/002, 2009 04 06 LT/1/09/1512/003 20090406; FIRST REGISTRATION: BE 327792 20081103 |
| 0771217 | 07C0001 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL BETADEX CLATHRATE; NAT. REGISTRATION NO/DATE: NL 32343 20060710; FIRST REGISTRATION: NL - RVG 31781 20050804 |
| 0398460 | 04C0022 | France | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL ANHYDRE DROSPIRENONE; REGISTRATION NO/DATE IN FRANCE: NL 28661 DU 20040316; REGISTRATION NO/DATE AT EEC: RVG 27505 DU 20021211 |
| 1214076 | C01214076/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE + ETHINYLESTRADIOL; REGISTRATION NUMBER/DATE: SWISSMEDIC 57946 13.06.2008 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.