Am Regent Company Profile
✉ Email this page to a colleague
What is the competitive landscape for AM REGENT, and what generic alternatives to AM REGENT drugs are available?
AM REGENT has seventy-eight approved drugs.
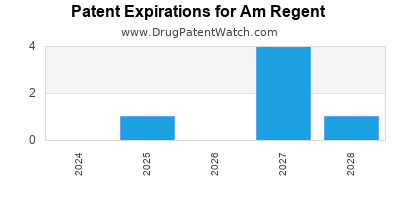
There are seven US patents protecting AM REGENT drugs.
There are sixty-seven patent family members on AM REGENT drugs in thirty-two countries and one hundred and forty-three supplementary protection certificates in fifteen countries.
Drugs and US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Am Regent | PAMIDRONATE DISODIUM | pamidronate disodium | INJECTABLE;INJECTION | 078942-001 | Jul 25, 2008 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Am Regent | BETAMETHASONE ACETATE AND BETAMETHASONE SODIUM PHOSPHATE | betamethasone acetate; betamethasone sodium phosphate | INJECTABLE;INJECTION | 090747-001 | Jul 31, 2009 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Am Regent | NANDROLONE DECANOATE | nandrolone decanoate | INJECTABLE;INJECTION | 091252-001 | Aug 30, 2010 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Am Regent
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-001 | Jul 25, 2013 | 10,519,252 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-004 | Feb 4, 2022 | 11,291,645 | ⤷ Try a Trial |
| Am Regent | INJECTAFER | ferric carboxymaltose | SOLUTION;INTRAVENOUS | 203565-003 | Apr 28, 2021 | 11,291,645 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for Am Regent Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Denmark | 1554315 | ⤷ Try a Trial |
| Canada | 2635894 | ⤷ Try a Trial |
| South Korea | 101905340 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Am Regent Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1507558 | 12C0033 | France | ⤷ Try a Trial | PRODUCT NAME: ALISKIRENE OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE E CELUI-CI, AMLODIPINE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI, ET HYDROCHLOROTHIAZIDE OU SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; NAT. REGISTRATION NO/DATE: EU/1/11/730/001 20111122; FIRST REGISTRATION: CH - 6167801 20110705 |
| 2957286 | CA 2018 00043 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PATIROMER SORBITEX CALCIUM AND ANY SALTS AND DERIVATIVES THEREOF; REG. NO/DATE: EU/1/17/1179/001-009 20170721 |
| 0136011 | 99C0003 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL AND NORETHISTERONE; FIRST REGISTRATION NO/DATE: 403 IS 106 F3 19980928; FIRST REGISTRATION: SE 14007 19980306 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.