PF PRISM CV Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PF PRISM CV, and what generic alternatives to PF PRISM CV drugs are available?
PF PRISM CV has sixteen approved drugs.
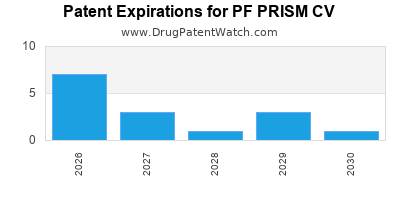
There are twenty-nine US patents protecting PF PRISM CV drugs.
There are six hundred and twelve patent family members on PF PRISM CV drugs in sixty-six countries and seventy-two supplementary protection certificates in eighteen countries.
Summary for PF PRISM CV
| International Patents: | 612 |
| US Patents: | 29 |
| Tradenames: | 11 |
| Ingredients: | 11 |
| NDAs: | 16 |
Drugs and US Patents for PF PRISM CV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pf Prism Cv | XALKORI | crizotinib | CAPSULE, PELLETS;ORAL | 217581-001 | Sep 7, 2023 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | CAPSULE;ORAL | 217729-002 | Sep 26, 2023 | RX | Yes | Yes | 7,767,678*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pf Prism Cv | INLYTA | axitinib | TABLET;ORAL | 202324-002 | Jan 27, 2012 | RX | Yes | Yes | 8,791,140 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pf Prism Cv | XALKORI | crizotinib | CAPSULE;ORAL | 202570-002 | Aug 26, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | CAPSULE;ORAL | 217729-002 | Sep 26, 2023 | RX | Yes | Yes | RE42376*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pf Prism Cv | BOSULIF | bosutinib monohydrate | TABLET;ORAL | 203341-003 | Oct 27, 2017 | RX | Yes | No | 7,919,625*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | RX | Yes | Yes | RE41783 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PF PRISM CV
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pf Prism Cv | ZMAX | azithromycin | FOR SUSPENSION, EXTENDED RELEASE;ORAL | 050797-001 | Jun 10, 2005 | 6,984,403 | ⤷ Try a Trial |
| Pf Prism Cv | ZMAX | azithromycin | FOR SUSPENSION, EXTENDED RELEASE;ORAL | 050797-001 | Jun 10, 2005 | 7,887,844 | ⤷ Try a Trial |
| Pf Prism Cv | VFEND | voriconazole | INJECTABLE;INTRAVENOUS | 021267-001 | May 24, 2002 | 5,773,443 | ⤷ Try a Trial |
| Pf Prism Cv | PRISTIQ | desvenlafaxine succinate | TABLET, EXTENDED RELEASE;ORAL | 021992-001 | Feb 29, 2008 | 7,291,347 | ⤷ Try a Trial |
| Pf Prism Cv | RAPAMUNE | sirolimus | TABLET;ORAL | 021110-003 | Feb 23, 2004 | 5,212,155*PED | ⤷ Try a Trial |
| Pf Prism Cv | VFEND | voriconazole | TABLET;ORAL | 021266-002 | May 24, 2002 | 5,364,938 | ⤷ Try a Trial |
| Pf Prism Cv | XELJANZ | tofacitinib citrate | TABLET;ORAL | 203214-002 | May 30, 2018 | 7,842,699 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for PF PRISM CV drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | For Injection | 200 mg/vial | ➤ Subscribe | 2008-09-12 |
| ➤ Subscribe | Oral Suspension | 40 mg/mL | ➤ Subscribe | 2010-10-08 |
| ➤ Subscribe | Injection | 25 mg/mL, 1.8 mL vial | ➤ Subscribe | 2011-05-25 |
| ➤ Subscribe | Tablets | 0.5 mg | ➤ Subscribe | 2010-08-25 |
| ➤ Subscribe | Extended-release Tablets | 25 mg | ➤ Subscribe | 2015-05-08 |
| ➤ Subscribe | Tablets | 1 mg and 5 mg | ➤ Subscribe | 2018-02-23 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2018-10-15 |
| ➤ Subscribe | Tablets | 50 mg and 200 mg | ➤ Subscribe | 2008-04-14 |
| ➤ Subscribe | For Injection | 50 mg per vial | ➤ Subscribe | 2009-06-15 |
| ➤ Subscribe | Tablets | 1 mg and 2 mg | ➤ Subscribe | 2009-12-17 |
| ➤ Subscribe | Extended-release Tablets | 50 mg and 100 mg | ➤ Subscribe | 2012-02-29 |
| ➤ Subscribe | Tablets | 0.5 mg and 1 mg | ➤ Subscribe | 2010-05-10 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2016-11-07 |
| ➤ Subscribe | Tablets | 100mg and 500mg | ➤ Subscribe | 2016-09-06 |
International Patents for PF PRISM CV Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| South Korea | 20090127949 | ⤷ Try a Trial |
| Poland | 378759 | ⤷ Try a Trial |
| European Patent Office | 1466889 | ⤷ Try a Trial |
| Eurasian Patent Organization | 200200120 | ⤷ Try a Trial |
| Australia | 2006323027 | ⤷ Try a Trial |
| Poland | 355907 | ⤷ Try a Trial |
| African Regional IP Organization (ARIPO) | 1486 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for PF PRISM CV Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1218348 | CA 2013 00010 | Denmark | ⤷ Try a Trial | |
| 0648494 | C300055 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SIROLIMUS, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AAN- VAARDBAAR ZOUT; NATL. REGISTRATION NO/DATE: EU/1/01/171/001 - EU/1/01/171/005 20010313; FIRST REGISTRATION: CH CH/55243 20000926 |
| 1786785 | 2013C/021 | Belgium | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), OTHER |
| 0401747 | 25/2001 | Austria | ⤷ Try a Trial | PRODUCT NAME: SIROLIMUS; NAT. REGISTRATION NO/DATE: EU/1/01/171/001 - EU/1/01/171/005 20010313; FIRST REGISTRATION: LI 5524301 20000926 |
| 1218348 | 122013000016 | Germany | ⤷ Try a Trial | PRODUCT NAME: AXITINIB, GEGEBENENFALLS IN FORM EINES PHARMAZEUTISCH ANNEHMBAREN SALZES; REGISTRATION NO/DATE: EU/1/12/777/001-006 20120903 |
| 1786785 | PA2013005 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: CRIZOTINIBUM; REGISTRATION NO/DATE: EU/1/12/793/001 - EU/1/12/793/004 20121023 |
| 1666481 | 2017C/032 | Belgium | ⤷ Try a Trial | PRODUCT NAME: TOFACITINIB; AUTHORISATION NUMBER AND DATE: EU/1/17/1178 20170324 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.