LUPIN LTD Company Profile
✉ Email this page to a colleague
What is the competitive landscape for LUPIN LTD, and when can generic versions of LUPIN LTD drugs launch?
LUPIN LTD has one hundred and fifty-eight approved drugs.
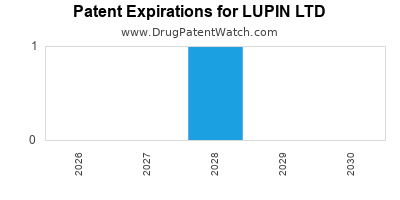
There is one US patent protecting LUPIN LTD drugs. There are nineteen tentative approvals on LUPIN LTD drugs.
There are two patent family members on LUPIN LTD drugs in two countries and five hundred and eighty-three supplementary protection certificates in seventeen countries.
Summary for LUPIN LTD
| International Patents: | 2 |
| US Patents: | 1 |
| Tradenames: | 137 |
| Ingredients: | 120 |
| NDAs: | 158 |
| Drug Master File Entries: | 194 |
| Patent Litigation for LUPIN LTD: | See patent lawsuits for LUPIN LTD |
| PTAB Cases with LUPIN LTD as petitioner: | See PTAB cases with LUPIN LTD as petitioner |
Drugs and US Patents for LUPIN LTD
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lupin Ltd | BLISOVI FE 1.5/30 | ethinyl estradiol; norethindrone acetate | TABLET;ORAL-28 | 201585-001 | Nov 18, 2015 | BX | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | MELOXICAM | meloxicam | CAPSULE;ORAL | 209487-001 | Jun 1, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | LEVETIRACETAM | levetiracetam | TABLET, EXTENDED RELEASE;ORAL | 091399-001 | Sep 12, 2011 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Lupin Ltd | MOXIFLOXACIN HYDROCHLORIDE | moxifloxacin hydrochloride | SOLUTION/DROPS;OPHTHALMIC | 204079-001 | May 28, 2015 | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Paragraph IV (Patent) Challenges for LUPIN LTD drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Injection | 250 mcg/0.5 mL, 1 mL PFS | ➤ Subscribe | 2012-03-30 |
| ➤ Subscribe | for Oral Suspension | 500 mg/5 mL | ➤ Subscribe | 2014-07-22 |
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
International Patents for LUPIN LTD Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2007119249 | ⤷ Try a Trial |
| Germany | 112007000920 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for LUPIN LTD Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2435025 | 19C1040 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE GLYCOPYRROLATE (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI) ET DE FORMOTEROL (Y COMPRIS TOUS SELS, ESTERS, ENANTIOMERES PHARMACEUTIQUEMENT ACCEPTABLES DE CELUI-CI); NAT. REGISTRATION NO/DATE: EU/1/18/1339 20181220; FIRST REGISTRATION: - EU/1/18/1339 20181220 |
| 1559427 | 300599 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: MIRABEGRON EN ZOUTEN ERVAN; REGISTRATION NO/DATE: EU/1/12/809/001-014 20130107 |
| 1948158 | 122016000038 | Germany | ⤷ Try a Trial | PRODUCT NAME: SACUBITRIL/VALSARTAN, ALS SACUBITRIL VALSARTAN NATRIUM SALZKOMPLEX, D.H. TRINATRIUM-(3-((1 S,3R)-1-BIPHENYL-4-YLMETHYL-3-ETHOXYCARBONYL-1-BUTYLCARBAMOYL)PROPIONAT-(S)-3'-METHYL-2'-(PENTANOYL-(2"-(TETRAZOL-5-YLAT)BIPHENYL-4'-YLMETHYL)AMINO)BUTYRAT)-HEMIPENTAHYDRAT.; REGISTRATION NO/DATE: EU/1/15/1058 20151119 |
| 1663240 | 1590057-4 | Sweden | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF RILPIVIRINE, OR A PHARMACEUTICAL LY ACCEPTABLE SALT OF RILPIVIRINE, INCLUDING THE HYDROCHLORIDE SALT OF RILPIVIRINE, AND TENOFOVIR DISOPROXIL, IN PARTICULAR TENOFOVIR DISOPROXIL FUMARATE, AND EMTRICITABINE; REG. NO/DATE: EU/1/11/737 20111128 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.