synjardy xr Drug Patent Profile
✉ Email this page to a colleague
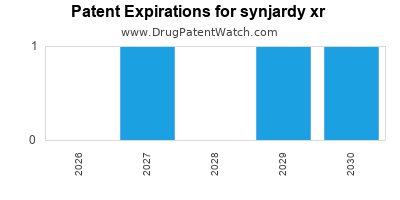
When do Synjardy Xr patents expire, and when can generic versions of Synjardy Xr launch?
Synjardy Xr is a drug marketed by Boehringer Ingelheim and is included in one NDA. There are nine patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and eight patent family members in forty-one countries.
The generic ingredient in SYNJARDY XR is empagliflozin; metformin hydrochloride. There are twenty-two drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the empagliflozin; metformin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Synjardy Xr
Synjardy Xr was eligible for patent challenges on August 1, 2018.
There have been fourteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for synjardy xr
| International Patents: | 208 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Clinical Trials: | 1 |
| Patent Applications: | 5 |
| Formulation / Manufacturing: | see details |
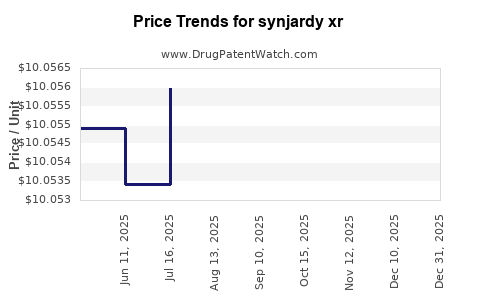
| Drug Prices: | Drug price information for synjardy xr |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for synjardy xr |
| DailyMed Link: | synjardy xr at DailyMed |
Recent Clinical Trials for synjardy xr
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hikma Pharma | Phase 1 |
| Genuine Research Center, Egypt | Phase 1 |
Pharmacology for synjardy xr
| Drug Class | Biguanide Sodium-Glucose Cotransporter 2 Inhibitor |
| Mechanism of Action | Sodium-Glucose Transporter 2 Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for synjardy xr
Paragraph IV (Patent) Challenges for SYNJARDY XR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| SYNJARDY XR | Extended-release Tablets | empagliflozin; metformin hydrochloride | 5 mg/1000 mg 10 mg/1000 mg 12.5 mg/1000 mg 25 mg/1000 mg | 208658 | 3 | 2018-08-01 |
US Patents and Regulatory Information for synjardy xr
synjardy xr is protected by ten US patents and two FDA Regulatory Exclusivities.
Patents protecting synjardy xr
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD FOR REDUCING THE RISK OF CARDIOVASCULAR DEATH AND HOSPITALIZATION FOR HEART FAILURE IN PATIENTS WITH HEART FAILURE AND TYPE 2 DIABETES MELLITUS BY ADMINISTRATION OF EMPAGLIFLOZIN
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR IS >=30 ML/MIN/1.73 M2 AND
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATING TYPE 2 DIABETES MELLITUS BY ASSESSING RENAL FUNCTION AND ORALLY ADMINISTERING EMPAGLIFLOZIN IN A DAILY AMOUNT OF 10 MG OR 25 MG IF THE EGFR>=45 ML/MIN/1.73 M2 AND
Glucopyranosyl-substituted phenyl derivatives, medicaments containing such compounds, their use and process for their manufacture
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystalline form of 1-chloro-4-(.beta.-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy- )-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical composition, methods for treating and uses thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting synjardy xr
REVISIONS TO THE LABELING TO INCLUDE RESULTS FROM PROTOCOL 1218- 0091
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
Expired US Patents for synjardy xr
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim | SYNJARDY XR | empagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 208658-003 | Dec 9, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | SYNJARDY XR | empagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 208658-001 | Dec 9, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | SYNJARDY XR | empagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 208658-004 | Dec 9, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Boehringer Ingelheim | SYNJARDY XR | empagliflozin; metformin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 208658-002 | Dec 9, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for synjardy xr
See the table below for patents covering synjardy xr around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20210131442 | 엠파글리플로진의 치료적 용도 (THERAPEUTIC USES OF EMPAGLIFLOZIN) | ⤷ Try a Trial |
| Taiwan | I344465 | ⤷ Try a Trial | |
| Portugal | 1888552 | ⤷ Try a Trial | |
| Hong Kong | 1115133 | ⤷ Try a Trial | |
| Ukraine | 89040 | ЗАМЕЩЕННЫЕ ГЛЮКОПИРАНОЗИЛОМ БЕНЗОЛЬНЫЕ ПРОИЗВОДНЫЕ, ЛЕКАРСТВЕННОЕ СРЕДСТВО, КОТОРОЕ СОДЕРЖИТ ЭТИ СОЕДИНЕНИЯ;ЗАМІЩЕНІ ГЛЮКОПІРАНОЗИЛОМ БЕНЗОЛЬНІ ПОХІДНІ, ЛІКАРСЬКИЙ ЗАСІБ, ЯКИЙ МІСТИТЬ ЦІ СПОЛУКИ (GLUCOPYRANOSYL-SUBSTITUTED BENZOL DERIVATIVES, DRUGS CONTAINING SAID COMPOUNDS) | ⤷ Try a Trial |
| European Patent Office | 4119136 | UTILISATIONS THÉRAPEUTIQUES D'EMPAGLIFLOZINE (THERAPEUTIC USES OF EMPAGLIFLOZIN) | ⤷ Try a Trial |
| Mexico | 2020013740 | USO DE EMPAGLIFLOZINA PARA EL TRATAMIENTO DE TRASTORNOS METABOLICOS, LA PREVENCION, Y PARA LA REDUCCION DEL RIESGO DE SUFRIR O PARA RETRASAR LA OCURRENCIA DE UN EPISODIO CARDIOVASCULAR. (THERAPEUTIC USES OF EMPAGLIFLOZIN.) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for synjardy xr
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1730131 | SPC/GB14/070 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN AND SALTS THEREOF; REGISTERED: UK EU/1/14/930/001 - 20140527; UK EU/1/14/930/018 20140527 |
| 1730131 | 200 5026-2014 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN; REGISTRATION NO/DATE: EU/1/14/930 20140527 |
| 1730131 | C01730131/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN; REGISTRATION NO/DATE: SWISSMEDIC 63227 12.11.2014 |
| 1730131 | C20140033 00134 | Estonia | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOSIIN;REG NO/DATE: EU/1/14/930 27.05.2014 |
| 1730131 | C01730131/02 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN UND METFORMINHYDROCHLORID; REGISTRATION NO/DATE: SWISSMEDIC 65570 12.11.2015 |
| 1730131 | PA2014035,C1730131 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZINUM; REGISTRATION NO/DATE: EU/1/14/930 20140522 |
| 1730131 | 2014/055 | Ireland | ⤷ Try a Trial | PRODUCT NAME: EMPAGLIFLOZIN AND SALTS THEREOF, IN PARTICULAR EMPAGLIFLOZIN ((1S)-1,5- ANHYDRO-1-C-(4-CHLORO-3-((4-(((3S)-OXOLAN-3-YL)OXY))PHENYL)METHYL)PHENYL)- D-GLUCITOL); REGISTRATION NO/DATE: EU/1/14/930 20140522 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


