halaven Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Halaven, and what generic alternatives are available?
Halaven is a drug marketed by Eisai Inc and is included in one NDA. There are two patents protecting this drug and one Paragraph IV challenge.
This drug has seventy-eight patent family members in twenty-six countries.
The generic ingredient in HALAVEN is eribulin mesylate. Two suppliers are listed for this compound. Additional details are available on the eribulin mesylate profile page.
DrugPatentWatch® Generic Entry Outlook for Halaven
Halaven was eligible for patent challenges on November 15, 2014.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 8, 2027. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for halaven
| International Patents: | 78 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 31 |
| Clinical Trials: | 62 |
| Patent Applications: | 7 |
| Drug Prices: | Drug price information for halaven |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for halaven |
| What excipients (inactive ingredients) are in halaven? | halaven excipients list |
| DailyMed Link: | halaven at DailyMed |
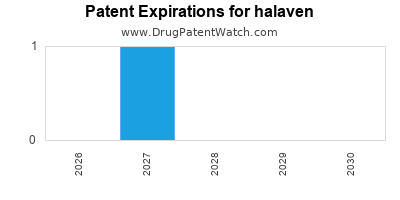

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for halaven
Generic Entry Date for halaven*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for halaven
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Australia New Zealand Gynaecological Oncology Group | Phase 2 |
| Merck Sharp & Dohme LLC | Phase 2 |
| Criterium, Inc. | Phase 2 |
Pharmacology for halaven
| Drug Class | Microtubule Inhibitor |
| Physiological Effect | Microtubule Inhibition |
Anatomical Therapeutic Chemical (ATC) Classes for halaven
Paragraph IV (Patent) Challenges for HALAVEN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| HALAVEN | Injection | eribulin mesylate | 1 mg/2 mL | 201532 | 1 | 2019-12-20 |
US Patents and Regulatory Information for halaven
halaven is protected by two US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of halaven is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting halaven
Macrocyclic analogs and methods of their use and preparation
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Intermediates for the preparation of analogs of Halichondrin B
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting halaven
REVISIONS TO THE PEDIATRIC USE SUBSECTION OF LABELING TO INCLUDE THE RESULTS FROM CLINICAL STUDIES E7389-G000-223 AND E7389-G000-213, CONDUCTED TO FULFILL A PEDIATRIC WRITTEN REQUEST
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for halaven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | HALAVEN | eribulin mesylate | SOLUTION;INTRAVENOUS | 201532-001 | Nov 15, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for halaven
When does loss-of-exclusivity occur for halaven?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 05250487
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 67984
Estimated Expiration: ⤷ Try a Trial
Patent: 22994
Estimated Expiration: ⤷ Try a Trial
Patent: 35786
Estimated Expiration: ⤷ Try a Trial
Patent: 28453
Estimated Expiration: ⤷ Try a Trial
China
Patent: 93342
Estimated Expiration: ⤷ Try a Trial
Patent: 1899026
Estimated Expiration: ⤷ Try a Trial
Patent: 4876896
Estimated Expiration: ⤷ Try a Trial
Patent: 8997267
Estimated Expiration: ⤷ Try a Trial
Patent: 9180615
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0150543
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 71431
Estimated Expiration: ⤷ Try a Trial
Patent: 22663
Estimated Expiration: ⤷ Try a Trial
Patent: 49652
Estimated Expiration: ⤷ Try a Trial
Patent: 87408
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 46436
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 0252
Estimated Expiration: ⤷ Try a Trial
Patent: 7402
Estimated Expiration: ⤷ Try a Trial
Patent: 7474
Estimated Expiration: ⤷ Try a Trial
Patent: 7620
Estimated Expiration: ⤷ Try a Trial
Patent: 7606
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 26304
Estimated Expiration: ⤷ Try a Trial
Patent: 05814
Estimated Expiration: ⤷ Try a Trial
Patent: 64926
Estimated Expiration: ⤷ Try a Trial
Patent: 38316
Estimated Expiration: ⤷ Try a Trial
Patent: 06235
Estimated Expiration: ⤷ Try a Trial
Patent: 08501715
Estimated Expiration: ⤷ Try a Trial
Patent: 13056923
Estimated Expiration: ⤷ Try a Trial
Patent: 15061845
Estimated Expiration: ⤷ Try a Trial
Patent: 16183171
Estimated Expiration: ⤷ Try a Trial
Patent: 17141241
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 22663
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 054
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 22663
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1434673
Estimated Expiration: ⤷ Try a Trial
Patent: 070030260
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 48200
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering halaven around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2949652 | Intermédiaires pour la préparation d'halichondrine B (Intermediates for the preparation of halichondrin B) | ⤷ Try a Trial |
| Slovenia | 2522663 | ⤷ Try a Trial | |
| Russian Federation | 2245335 | МАКРОЦИКЛИЧЕСКОЕ СОЕДИНЕНИЕ И СПОСОБ ИДЕНТИФИКАЦИИ АГЕНТА НА ЕГО ОСНОВЕ (MACROCYCLIC COMPOUND AND METHOD FOR IDENTIFYING AGENT BASED ON THEREOF) | ⤷ Try a Trial |
| Brazil | PI9911326 | análogos macrocíclicos e métodos para seu uso e preparação | ⤷ Try a Trial |
| European Patent Office | 2272839 | Intermédiaires pour la préparation d'analogues d'halichondrine (Intermediate compounds for the preparation of halichondrin analogs) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for halaven
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1087960 | 300493 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ERIBULIN, ALSMEDE FARMACEUTISCHE ZOUTEN ERVAN, IN HET BIJZONDER HET MESYLAAT; REGISTRATION NO/DATE: EU/1/11/678/001-002 20110317 |
| 1087960 | 394 | Finland | ⤷ Try a Trial | |
| 1087960 | 122011100031 | Germany | ⤷ Try a Trial | PRODUCT NAME: ERIBULIN UND PHARMAZEUTISCH ANNEHMBARE SALZE HIERVON, INSBESONDERE ERIBULINMESYLAT; REGISTRATION NO/DATE: EU/1/11/678/001-002 20110317 |
| 1087960 | 91854 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: ERIBULIN ET SES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES (HALAVEN ); AUTHORISATION NUMBER AND DATE: EU/1/11/678/001-002, 17.03.2011 |
| 1087960 | SPC026/2011 | Ireland | ⤷ Try a Trial | SPC026/2011: 20111128, EXPIRES: 20240615 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


