XARELTO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Xarelto, and when can generic versions of Xarelto launch?
Xarelto is a drug marketed by Janssen Pharms and is included in two NDAs. There are four patents protecting this drug and three Paragraph IV challenges.
This drug has one hundred and fifty-six patent family members in forty-seven countries.
The generic ingredient in XARELTO is rivaroxaban. There are thirty-five drug master file entries for this compound. Nineteen suppliers are listed for this compound. Additional details are available on the rivaroxaban profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Xarelto
A generic version of XARELTO was approved as rivaroxaban by LUPIN LTD on March 3rd, 2025.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for XARELTO?
- What are the global sales for XARELTO?
- What is Average Wholesale Price for XARELTO?
Summary for XARELTO
| International Patents: | 156 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 4 |
| Raw Ingredient (Bulk) Api Vendors: | 92 |
| Clinical Trials: | 128 |
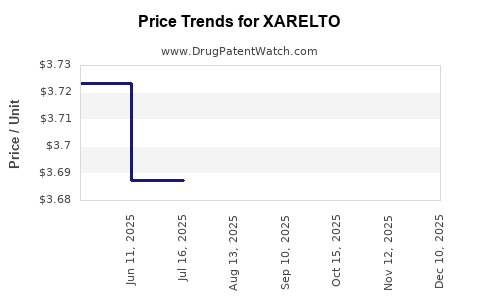
| Drug Prices: | Drug price information for XARELTO |
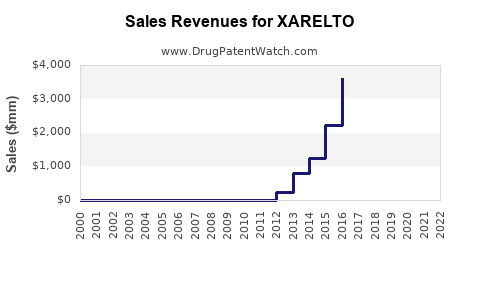
| Drug Sales Revenues: | Drug sales revenues for XARELTO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for XARELTO |
| What excipients (inactive ingredients) are in XARELTO? | XARELTO excipients list |
| DailyMed Link: | XARELTO at DailyMed |
Recent Clinical Trials for XARELTO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| U.S. Food and Drug Administration (FDA) | PHASE4 |
| VA Office of Research and Development | PHASE4 |
| Prof. Stavros Konstantinides, MD | PHASE3 |
Pharmacology for XARELTO
| Drug Class | Factor Xa Inhibitor |
| Mechanism of Action | Factor Xa Inhibitors |
Paragraph IV (Patent) Challenges for XARELTO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| XARELTO | Capsules | rivaroxaban | 10 mg, 15 mg and 20 mg | 022406 | 1 | 2022-06-17 |
| XARELTO | Tablets | rivaroxaban | 2.5 mg | 022406 | 4 | 2018-11-19 |
| XARELTO | Tablets | rivaroxaban | 10 mg, 15 mg, and 20 mg | 022406 | 8 | 2015-07-01 |
US Patents and Regulatory Information for XARELTO
XARELTO is protected by four US patents and four FDA Regulatory Exclusivities.
Expired US Patents for XARELTO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-001 | Jul 1, 2011 | 7,592,339 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-002 | Nov 4, 2011 | 7,592,339 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-004 | Oct 11, 2018 | 7,585,860 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-002 | Nov 4, 2011 | 7,585,860 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-004 | Oct 11, 2018 | 7,592,339 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-001 | Jul 1, 2011 | 7,585,860 | ⤷ Get Started Free |
| Janssen Pharms | XARELTO | rivaroxaban | TABLET;ORAL | 022406-003 | Nov 4, 2011 | 7,592,339 | ⤷ Get Started Free |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for XARELTO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Accord Healthcare S.L.U. | Rivaroxaban Accord | rivaroxaban | EMEA/H/C/005279Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery.Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4.4 for haemodynamically unstable PE patients.Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4.4 for haemodynamically unstable PE patients).AdultsPrevention of stroke and systemic embolism in adult patients with non valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4.4 for haemodynamically unstable PE patients.)Paediatric populationTreatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.Rivaroxaban Accord, co administered with acetylsalicylic acid (ASA) alone or with ASA plus ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers (see sections 4.3, 4.4 and 5.1).Rivaroxaban Accord, co administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.AdultsPrevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. (See section 4.4 for haemodynamically unstable PE patients.)Paediatric populationTreatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment. | Authorised | yes | no | no | 2020-11-16 | |
| Mylan Ireland Limited | Rivaroxaban Viatris (previously Rivaroxaban Mylan) | rivaroxaban | EMEA/H/C/005600Rivaroxaban Mylan co-administered with acetylsalicylic acid (ASA) alone or with ASA plus clopidogrel or ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers. Rivaroxaban Mylan co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events. ------Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery. Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.-------Adults Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.Paediatric population Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.Paediatric population Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment. | Authorised | yes | no | no | 2021-11-12 | |
| Bayer AG | Xarelto | rivaroxaban | EMEA/H/C/000944Xarelto, co-administered with acetylsalicylic acid (ASA) alone or with ASA plus clopidogrel or ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome (ACS) with elevated cardiac biomarkers.Xarelto, co-administered with acetylsalicylic acid (ASA), is indicated for the prevention of atherothrombotic events in adult patients with coronary artery disease (CAD) or symptomatic peripheral artery disease (PAD) at high risk of ischaemic events.Prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery.Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults.AdultsPrevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack.Paediatric population Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing from 30 kg to 50 kg after at least 5 days of initial parenteral anticoagulation treatment.Paediatric population Treatment of venous thromboembolism (VTE) and prevention of VTE recurrence in children and adolescents aged less than 18 years and weighing more than 50 kg after at least 5 days of initial parenteral anticoagulation treatment. | Authorised | no | no | no | 2008-09-30 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for XARELTO
See the table below for patents covering XARELTO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| New Zealand | 519730 | Substituted oxazolidinones and their use in the field of blood coagulation and preparation process thereof | ⤷ Get Started Free |
| Canada | 2596145 | PREVENTION ET TRAITEMENT DE TROUBLES THROMBOEMBOLIQUES (PREVENTION AND TREATMENT OF THROMBOEMBOLIC DISORDERS) | ⤷ Get Started Free |
| Germany | 50009607 | ⤷ Get Started Free | |
| Taiwan | I277615 | ⤷ Get Started Free | |
| Israel | 175860 | שיטה ליצור תכשיר רוקחות מוצק למתן פומי (Method for the production of a solid, orally applicable pharmaceutical composition) | ⤷ Get Started Free |
| Singapore | 159505 | PREVENTION AND TREATMENT OF THROMBOEMBOLIC DISORDERS | ⤷ Get Started Free |
| Japan | 2007512274 | ⤷ Get Started Free | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for XARELTO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261606 | C 2008 019 | Romania | ⤷ Get Started Free | PRODUCT NAME: 5-CLORO-N-({(5S)-2-OXO-3-[4-(3-OXO-4-MORFOLINIL)FENIL]-1,3-OXAZOLIDIN-5-IL}-METIL)-2TIOFENCARBOXAMIDA - RIVAROXABAN; NATIONAL AUTHORISATION NUMBER: RO EU/1/08/472/001, RO EU/1/08/472/002, RO EU/1/08/472/003, RO EU/1/08/472/004, RO EU/1/08/472/005, RO EU/1/08/472/006, RO EU/1/08/472/007, RO EU/1/08/472/008; DATE OF NATIONAL AUTHORISATION: 20080930; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EMEA EU/1/08/472/001, EMEA EU/1/08/472/002, EMEA EU/1/08/472/003, EMEA EU/1/08/472/004, EMEA EU/1/08/472/005, EMEA EU/1/08/472/006, EMEA EU/1/08/472/007, EMEA EU/1/08/472/008; DATE OF FIRST AUTHORISATION IN EEA: 20080930 |
| 1261606 | CA 2008 00050 | Denmark | ⤷ Get Started Free | PRODUCT NAME: RIVAROXABAN OG DETS FARMACEUTISK ACCEPTABLE SALTE, HYDRATER, HYDRATER AF SALTENE OG PRODRUGS |
| 1261606 | PA2008018,C1261606 | Lithuania | ⤷ Get Started Free | PRODUCT NAME: RIVAROXABANUM; REGISTRATION NO/DATE: EU/1/07/472/001 - EU/1/08/472/008, 0080930 |
| 1261606 | 2008C/046 | Belgium | ⤷ Get Started Free | PRODUCT NAME: RIVAROXABAN; AUTHORISATION NUMBER AND DATE: EU/1/08/472/001 |
| 1261606 | SPC/GB09/008 | United Kingdom | ⤷ Get Started Free | SUPPLEMENTARY PROTECTION CERTIFICATE NO SPC/GB09/008 GRANTED TO BAYER INTELLECTUAL PROPERTY GMBH IN RESPECT OF THE PRODUCT RIVAROXABAN AND ITS PHARMACEUTICALLY ACCEPTABLE SALTS, HYDRATES, HYDRATES OF THE SALTS AND PRODRUGS, THE GRANT OF WHICH WAS ADVERTISED IN JOURNAL NO 6352 DATED 16 FEBRUARY 2011 HAS HAD ITS MAXIMUM PERIOD OF DURATION CORRECTED, SUBJECT TO THE PAYMENT OF THE PRESCRIBED FEES IT WILL EXPIRE ON 01 OCTOBER 2023. |
| 1261606 | 300370 | Netherlands | ⤷ Get Started Free | |
| 1261606 | 2008/030 | Ireland | ⤷ Get Started Free | PRODUCT NAME: RIVAROXABAN AND ITS PHARMACEUTICALLY ACCEPTABLE SALTS, HYDRATES, HYDRATES OF THE SALTS AND PRODRUGS.; REGISTRATION NO/DATE: EU/1/08/472/001-008 20080930 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Market Dynamics and Financial Trajectory for XARELTO (Rivaroxaban)
More… ↓
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. We do not provide individual investment advice. This service is not registered with any financial regulatory agency. The information we publish is educational only and based on our opinions plus our models. By using DrugPatentWatch you acknowledge that we do not provide personalized recommendations or advice. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.
