VITRAKVI Drug Patent Profile
✉ Email this page to a colleague
When do Vitrakvi patents expire, and when can generic versions of Vitrakvi launch?
Vitrakvi is a drug marketed by Bayer Hlthcare and Bayer Healthcare and is included in two NDAs. There are eighteen patents protecting this drug.
This drug has two hundred and eleven patent family members in fifty countries.
The generic ingredient in VITRAKVI is larotrectinib sulfate. Two suppliers are listed for this compound. Additional details are available on the larotrectinib sulfate profile page.
DrugPatentWatch® Generic Entry Outlook for Vitrakvi
Vitrakvi was eligible for patent challenges on November 26, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 15, 2036. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for VITRAKVI
| International Patents: | 211 |
| US Patents: | 18 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 91 |
| Clinical Trials: | 5 |
| Patent Applications: | 210 |
| Drug Prices: | Drug price information for VITRAKVI |
| What excipients (inactive ingredients) are in VITRAKVI? | VITRAKVI excipients list |
| DailyMed Link: | VITRAKVI at DailyMed |
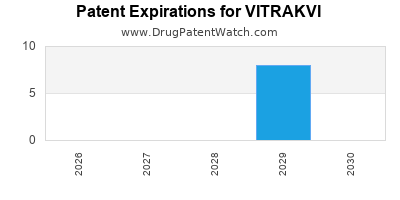

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for VITRAKVI
Generic Entry Dates for VITRAKVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for VITRAKVI*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for VITRAKVI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| M.D. Anderson Cancer Center | Phase 2 |
| Children's Oncology Group | Phase 2 |
| Bayer | Phase 2 |
Pharmacology for VITRAKVI
| Drug Class | Kinase Inhibitor |
| Mechanism of Action | Tropomyosin Receptor Kinases Inhibitors |
Anatomical Therapeutic Chemical (ATC) Classes for VITRAKVI
US Patents and Regulatory Information for VITRAKVI
VITRAKVI is protected by nineteen US patents and three FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of VITRAKVI is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting VITRAKVI
Method of treatment using substituted pyrazolo[1,5-a] pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING NEUROBLASTOMA, GLIOMA, THYROID, AND BREAST CANCER SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION
Methods of treating pediatric cancers
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING CMN, IFS, HGG, DIPGS, PTC, SOFT TISSUE SARCOMA, AND SPINDLE CELL SARCOMA SOLID TUMORS EXHIBITING AN NTRK GENE FUSION IN A PEDIATRIC PATIENT WITH AN ORAL SOLUTION
Method of treatment using substituted pyrazolo[1,5-a] pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION AFTER SURGICAL RESECTION
Liquid formulations of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION
Liquid formulations of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of treatment using substituted pyrazolo[1,5-A] pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-YL)-pyrazolo[1,5-A]pyri- midin-3-YL)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING LUNG CANCER, UNDIFFERENTIATED SARCOMA, OR COLORECTAL CANCER THAT EXHIBITS AN NTRK GENE FUSION
Methods of treating pediatric cancers
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK FUSION GENE IN A PEDIATRIC PATIENT
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION
Substituted pyrazolo[1,5-a]pyrimidine compounds as Trk kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of treatment using substituted pyrazolo[1,5-a]pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING CANCEROUS SOLID TUMORS
Method of treatment using substituted pyrazolo[1,5-a] pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method of treatment using substituted pyrazolo[1,5-a]pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION
Method of treatment using substituted pyrazolo[1,5-A] pyrimidine compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING CANCEROUS SOLID TUMORS
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK GENE FUSION IN A PEDIATRIC PATIENT
Crystalline form of (S)-N-(5-((R)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-A]pyri- midin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING SOLID TUMORS THAT EXHIBIT AN NTRK FUSION GENE IN A PEDIATRIC PATIENT
FDA Regulatory Exclusivity protecting VITRAKVI
INDICATED FOR THE TREATMENT OF ADULT AND PEDIATRIC PATIENTS WITH SOLID TUMORS THAT HAVE NO SATISFACTORY ALTERNATIVE TREATMENTS OR THAT HAVE PROGRESSED FOLLOWING TREATMENT
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF ADULT AND PEDIATRIC PATIENTS WITH SOLID TUMORS THAT HAVE A NEUROTROPHIC RECEPTOR TYROSINE KINASE (NTRK) GENE FUSION WITHOUT A KNOWN ACQUIRED RESISTANCE MUTATION
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF ADULT AND PEDIATRIC PATIENTS WITH SOLID TUMORS THAT ARE METASTATIC OR WHERE SURGICAL RESECTION IS LIKELY TO RESULT IN SEVERE MORBIDITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Healthcare | VITRAKVI | larotrectinib sulfate | SOLUTION;ORAL | 211710-001 | Nov 26, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bayer Healthcare | VITRAKVI | larotrectinib sulfate | SOLUTION;ORAL | 211710-001 | Nov 26, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Bayer Healthcare | VITRAKVI | larotrectinib sulfate | SOLUTION;ORAL | 211710-001 | Nov 26, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bayer Hlthcare | VITRAKVI | larotrectinib sulfate | CAPSULE;ORAL | 210861-002 | Nov 26, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bayer Healthcare | VITRAKVI | larotrectinib sulfate | SOLUTION;ORAL | 211710-001 | Nov 26, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for VITRAKVI
When does loss-of-exclusivity occur for VITRAKVI?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 8090
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 15346046
Estimated Expiration: ⤷ Try a Trial
Patent: 17246547
Estimated Expiration: ⤷ Try a Trial
Patent: 17246554
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2017010141
Estimated Expiration: ⤷ Try a Trial
Patent: 2018070017
Estimated Expiration: ⤷ Try a Trial
Patent: 2018070304
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 67951
Estimated Expiration: ⤷ Try a Trial
Patent: 19661
Estimated Expiration: ⤷ Try a Trial
Patent: 19671
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 17001249
Estimated Expiration: ⤷ Try a Trial
Patent: 18002806
Estimated Expiration: ⤷ Try a Trial
Patent: 18002807
Estimated Expiration: ⤷ Try a Trial
Patent: 19002238
Estimated Expiration: ⤷ Try a Trial
Patent: 19002239
Estimated Expiration: ⤷ Try a Trial
China
Patent: 7428760
Estimated Expiration: ⤷ Try a Trial
Patent: 9310694
Estimated Expiration: ⤷ Try a Trial
Patent: 9414442
Estimated Expiration: ⤷ Try a Trial
Patent: 3354649
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 17005483
Estimated Expiration: ⤷ Try a Trial
Patent: 18010761
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 170263
Estimated Expiration: ⤷ Try a Trial
Patent: 180501
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0230271
Estimated Expiration: ⤷ Try a Trial
Cuba
Patent: 180125
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 018000214
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 18083443
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 18380
Estimated Expiration: ⤷ Try a Trial
Patent: 39662
Estimated Expiration: ⤷ Try a Trial
Patent: 39663
Estimated Expiration: ⤷ Try a Trial
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Finland
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Georgia, Republic of
Patent: 0227339
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 44483
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 61448
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 2270
Estimated Expiration: ⤷ Try a Trial
Patent: 2003
Estimated Expiration: ⤷ Try a Trial
Patent: 2005
Estimated Expiration: ⤷ Try a Trial
Patent: 0905
Estimated Expiration: ⤷ Try a Trial
Patent: 4018
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 14834
Estimated Expiration: ⤷ Try a Trial
Patent: 57343
Estimated Expiration: ⤷ Try a Trial
Patent: 61602
Estimated Expiration: ⤷ Try a Trial
Patent: 17535550
Estimated Expiration: ⤷ Try a Trial
Patent: 19510827
Estimated Expiration: ⤷ Try a Trial
Patent: 19511575
Estimated Expiration: ⤷ Try a Trial
Patent: 21169496
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 17006412
Estimated Expiration: ⤷ Try a Trial
Patent: 18012163
Estimated Expiration: ⤷ Try a Trial
Patent: 18012165
Estimated Expiration: ⤷ Try a Trial
Patent: 20011079
Estimated Expiration: ⤷ Try a Trial
Morocco
Patent: 601
Estimated Expiration: ⤷ Try a Trial
Patent: 610
Estimated Expiration: ⤷ Try a Trial
Patent: 612
Estimated Expiration: ⤷ Try a Trial
Patent: 504
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 1909
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 1800102
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 181888
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 017500900
Estimated Expiration: ⤷ Try a Trial
Patent: 018502124
Estimated Expiration: ⤷ Try a Trial
Patent: 018502134
Estimated Expiration: ⤷ Try a Trial
Patent: 021550809
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 23990
Estimated Expiration: ⤷ Try a Trial
Patent: 51636
Estimated Expiration: ⤷ Try a Trial
Patent: 51767
Estimated Expiration: ⤷ Try a Trial
Patent: 17120846
Estimated Expiration: ⤷ Try a Trial
Patent: 18137206
Estimated Expiration: ⤷ Try a Trial
Patent: 18138579
Estimated Expiration: ⤷ Try a Trial
Saudi Arabia
Patent: 8400168
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 122
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201703962X
Estimated Expiration: ⤷ Try a Trial
Patent: 201808559P
Estimated Expiration: ⤷ Try a Trial
Patent: 201808676R
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 99181
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1703501
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2400423
Estimated Expiration: ⤷ Try a Trial
Patent: 170082628
Estimated Expiration: ⤷ Try a Trial
Patent: 180128484
Estimated Expiration: ⤷ Try a Trial
Patent: 180129911
Estimated Expiration: ⤷ Try a Trial
Patent: 210010652
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 41630
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 46426
Estimated Expiration: ⤷ Try a Trial
Patent: 46537
Estimated Expiration: ⤷ Try a Trial
Patent: 67858
Estimated Expiration: ⤷ Try a Trial
Patent: 1625636
Estimated Expiration: ⤷ Try a Trial
Patent: 1801730
Estimated Expiration: ⤷ Try a Trial
Patent: 2206436
Estimated Expiration: ⤷ Try a Trial
Tunisia
Patent: 18000335
Estimated Expiration: ⤷ Try a Trial
Patent: 18000338
Estimated Expiration: ⤷ Try a Trial
Patent: 19000332
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 3044
Estimated Expiration: ⤷ Try a Trial
Patent: 5025
Estimated Expiration: ⤷ Try a Trial
Patent: 5026
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering VITRAKVI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 3439662 | FORMULATIONS LIQUIDES DE (S)-N-(5-((R)-2-(2,5-DIFLUOROPHÉNYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE (LIQUID FORMULATIONS OF (S)-N-(5-((R)-2-(2,5-DIFLUOROPHENYL)-PYRROLIDIN-1-YL)-PYRAZOLO[1,5-A]PYRIMIDIN-3-YL)-3-HYDROXYPYRROLIDINE-1-CARBOXAMIDE) | ⤷ Try a Trial |
| Taiwan | I767858 | ⤷ Try a Trial | |
| Israel | 252270 | צורה גבישית של (s)-n-(5-)-2-(r)) 5,2-דיפלואורופניל)-פירולידין-1-איל)-פיראזולו[5,1-a]פירימידין-3-איל)-3-הידרוקסיפירולידין-1-קרבוקסאמיד מימן סולפאט (Crystalline form of (s)-n-(5-((r)-2-(2,5-difluorophenyl)-pyrrolidin-1-yl)-pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide hydrogen sulfate) | ⤷ Try a Trial |
| Croatia | P20211722 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2010048314 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for VITRAKVI
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3106463 | C03106463/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: LAROTRECTINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67282 28.05.2020 |
| 3106463 | CR 2020 00013 | Denmark | ⤷ Try a Trial | PRODUCT NAME: LAROTRECTINIB OG/ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF, SAERLIGT LAROTRECTINIBSULFAT, INKLUSIV LAROTRECTINIBHYDROGENSULFAT; REG. NO/DATE: EU/1/19/1385 20190923 |
| 3106463 | 2020004 | Norway | ⤷ Try a Trial | PRODUCT NAME: LAROTREKTINIB OG/ELLER FARMASOEYTISK AKSEPTABLE SALTER DERAV, SAERLIG LAROTREKTINIBSULFAT INKLUDERT LAROTREKTINIBHYDROGENSULFAT; REG. NO/DATE: EU/1/19/1385 20191018 |
| 3106463 | PA2020504 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: LAROTREKTINIBAS IR (ARBA) FARMACINIU POZIURIU PRIIMTINOS DRUSKOS, YPAC LAROTREKTINIBO SULFATAS, ISKAITANT LAROTREKTINIBO VANDENILIO SULFATA; REGISTRATION NO/DATE: EU/1/19/1385 20190919 |
| 3106463 | 11/2020 | Austria | ⤷ Try a Trial | PRODUCT NAME: LAROTRECTINIB UND/ODER PHARMAZEUTISCH AKZEPTABLE SALZE DAVON, INSBESONDERE LAROTRECTINIBSULFAT, EINSCHLIESSLICH LAROTRECTINIBHYDROGENSULFAT; REGISTRATION NO/DATE: EU/1/19/1385 20190923 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


